All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Real-world treatment patterns and outcomes with luspatercept in patients with lower-risk myelodysplastic syndrome
Myelodysplastic syndromes (MDS) are characterized by inadequate hematopoiesis resulting in anemia and risk of progression to acute myeloid leukemia. Most patients with lower-risk MDS (LR-MDS) receive erythropoiesis-stimulating agents (ESAs); however, 50% of these patients do not respond and will experience disease progression within 8 weeks and will eventually require red blood cell (RBC) transfusion. Therefore, treatment options are limited for this patient population.1,2,3
Luspatercept is a U.S. FDA approved first-in class erythroid maturation agent for the management of anemia in patients with LR-MDS with ring sideroblasts (RS) or MDS-RS and thrombocytosis (MDS-RS-T) after ESA failure. It has also recently been granted FDA approval as a first-line treatment for management of anemia in patients with LR-MDS. However, there is paucity of data on the treatment patterns and outcomes of patients with LR-MDS treated with luspatercept in routine clinical practice.1,2,3
The MDS Hub has reported results from PACE-MDS and COMMANDS trial on the use of luspatercept in patients with LR-MDS. Here, we summarize a retrospective study presented by Mukerjee at the 2023 American Society of Clinical Oncology Annual Meeting and the European Hematology Association 2023 Congress, on treatment patterns and clinical outcomes among patients with LR-MDS treated with luspatercept.1,2 In addition, we also summarize the key findings from a real-world study on efficacy and safety of luspatercept and predictive factors of response in patients with LR-MDS with RS published by Lanino, et al. in American Journal of Hematology.3
Treatment patterns and outcomes of luspatercept1,2
Study design
This is a retrospective, observational, multicenter US based study in patients aged ≥18 years, diagnosed with LR-MDS, LR-MDS with RS, or MDS-RS-T who had an International Prognostic Scoring System score of 0–1.
Transfusion burden (TB) during luspatercept treatment was defined by the lowest number of transfusion sessions during a consecutive 8-week period as follows:
- Transfusion independence (TI) = 0 session
- Low TB = 1−3 sessions
- Moderate TB = 4−5 sessions
- High TB= ≥6 sessions.
Results
Baseline characteristics
A total of 253 patients were included, with a mean age of 73.2 and 71.3 years at luspatercept initiation and at diagnosis, respectively. The median duration of follow-up from luspatercept initiation was 5.7 months (range, 4.1–10.6 months). Selected baseline characteristics are summarized in Table 1.
Table 1. Selected baseline characteristics*
|
del, deletion; IPSS, International Prognostic Scoring System; IPSS-R, International Prognostic Scoring System revised; LR-MDS, lower-risk myelodysplastic syndromes; MDS-RS-T, myelodysplastic syndromes with ring sideroblasts and thrombocytosis; MPN, myeloproliferative neoplasm; NTD, non-transfusion dependent; RS, ring sideroblasts; TB, transfusion burden; TI, transfusion independence. *Data from Mukherjee, et al.1,2 |
|
|
Characteristic, % (unless otherwise stated) |
Patients |
|---|---|
|
Sex, male |
52.6 |
|
Diagnosis |
|
|
LR-MDS |
10.3 |
|
LR-MDS with RS |
85.8 |
|
MDS/MPN-RS-T |
4.0 |
|
IPSS/IPSS-R score at diagnosis |
|
|
Low/very low |
84.2 |
|
Intermediate |
15.8 |
|
Cytogenetic abnormalities |
|
|
-7/del(7q) |
4.0 |
|
del(5q) |
6.3 |
|
Complex karyotype |
5.5 |
|
Comorbidities at luspatercept initiation |
|
|
Cardiovascular disease |
34.4 |
|
Chronic pulmonary disease |
17.8 |
|
Diabetes |
37.9 |
|
Renal disease |
9.1 |
|
Baseline TB (8 weeks prior) |
|
|
NTD |
8.3 |
|
Low TB |
82.2 |
|
Moderate TB |
9.5 |
Treatment patterns
- Overall, 1.2%, 86.6% and 9.5% of patients received luspatercept as first-, second- and third-line treatment, respectively.
- Additionally, 87% of patients received treatment with an ESA prior to luspatercept initiation.
- The most common second-line treatment in patients who received ≥2 treatments prior to luspatercept were single-agent ESAs and hypomethylating agents in 29% (9/31) and 25.8% (8/31) of patients, respectively.
- Among 250 patients who received ≥1 treatment prior luspatercept, the most common reasons for discontinuation of first-line treatment were:
- Lack of improvement in anemia (53.2%);
- Disease progression (29.6%); and
- Completion of scheduled treatment (8.0%).
- In total 33.2% of patients discontinued luspatercept during the study and the reasons for discontinuation are shown in Figure 1.
- The median duration of luspatercept treatment was 10.8 months.
Figure 1. Primary reasons for discontinuation of luspatercept*
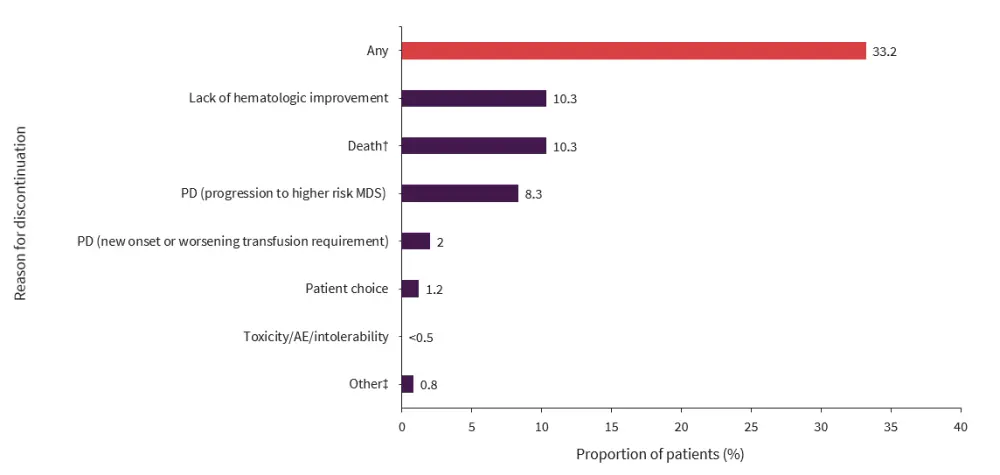
AE, adverse event; MDS, myelodysplastic syndrome; PD, progressive disease.
*Data from Mukherjee, et al.1,2
†Causes of death included COVID-19 (3.6%), PD (1.2%), and other (2.8%).
‡Included normalization of hemoglobin and progression to acute myeloid leukemia (<0.5% each).
Transfusion and hematologic outcomes
- During the first 24 weeks of luspatercept treatment in patients:
- The median hemoglobin level increased from 8.0 to 9.3 g/dL.
- The median absolute neutrophil count was maintained between 1,910 and 2,530 x109/L; and the median platelet count was maintained between 157 and 167 x 109/L.
- Of the 232 patients with transfusion dependence at baseline, 60.3% of patients achieved physician reported modified hematologic improvement-erythroid (mHI-E), 24.1% did not achieve mHI-E, and 15.5% of patients had an unknown mHI-E status.
- High rates of TB reduction and TI were achieved in 90.9% and 86.6% of patients with low or moderate TB at baseline.
- Most patients with low TB at baseline achieved TI during the first 24 weeks of luspatercept treatment (Figure 2).
- At ≥8 weeks, 20/21 patients who were non-transplant dependent at baseline continued to maintain TI.
Figure 2. Insfusion outcomes during Weeks 1–24 of luspatercept treatment*†
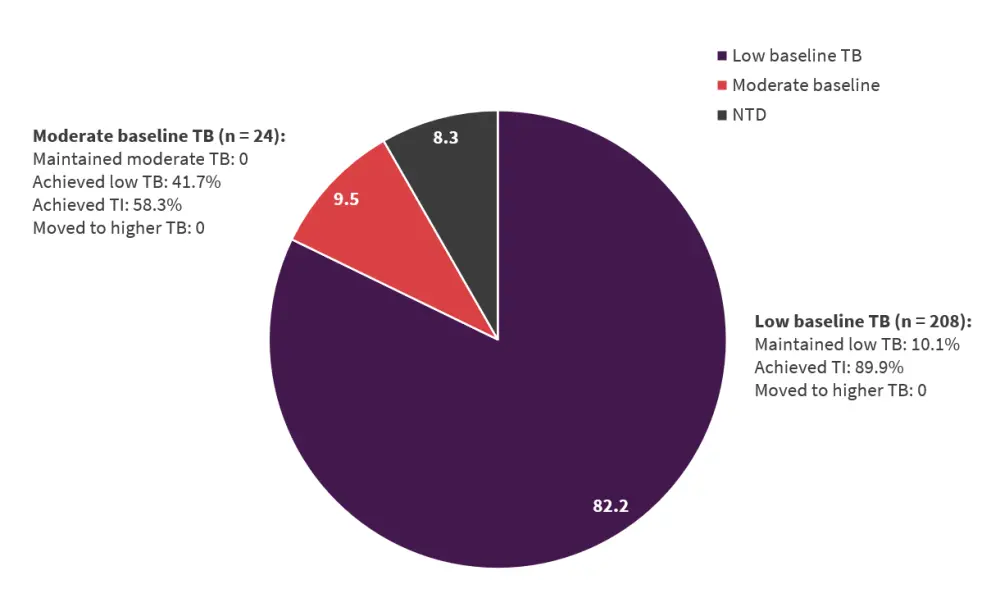
NTD, non-transfusion dependent; TB, transfusion burden; TI, transfusion independence.
*Data from Mukherjee.1,2
†Patients who were transfusion dependent at baseline 8 weeks prior to luspatercept treatment
Real-world data on efficacy and safety of luspatercept 3
Study design
Fondazione Italiana Sindromi Mielodisplastiche (FISiM) is a multicenter, observational trial (NCT05520749) in patients:
- aged ≥18 years diagnosed with MDS-RS according to the 2016 World Health Organization criteria;
- with an International Prognostic Scoring System score of very low, low, or intermediate risk;
- who had received regular RBC transfusions defined as ≥2 units/8 weeks during enrollment;
- were refractory to or unlikely to respond to ESA therapy; and
- treated with luspatercept.
The primary endpoint was TI for ≥8 weeks during Weeks 1−24 of luspatercept treatment. Key secondary endpoints were TI for ≥12 weeks during Weeks 1−24 and 1−48 of luspatercept treatment.
Results
Baseline characteristics
At data cut off, a total of 201 patients were included in this analysis. The median age at enrollment was 74 years (range, 31–89 years) and the median time since the first RBC transfusion was 21 months (range, 2−156 months). At least one comorbidity requiring ongoing treatment was present in 66.7% of patients, and ≥3 comorbidities in 21.4% of patients. The median RBC transfusion burden was 7 units/8 weeks (range, 2–22), and the median pre-transfusion hemoglobin was 7.9 (range, 5.55−9.6 g/dL).
Efficacy
- The median duration of TI was 23.9 weeks.
- TI for ≥8 and ≥12 weeks during Weeks 1–24 and 1–48, respectively are shown in Figure 3.
- Multivariate analyses showed a significant association between baseline TB and individual probability to achieve TI (p<0.001).
- The erythroid response according to the International Working Group 2006 criteria during Weeks 1–24 was achieved in 35.3% of patients; and a major erythroid response according to the International Working Group 2018 criteria in 6.9% of patients.
- A mean increase in the hemoglobin level of ≥1.5g/dL was observed in 13.9% and 21.9% of patients in the first 24 and 48 weeks, respectively.
Figure 3. Transfusion independence with luspatercept*
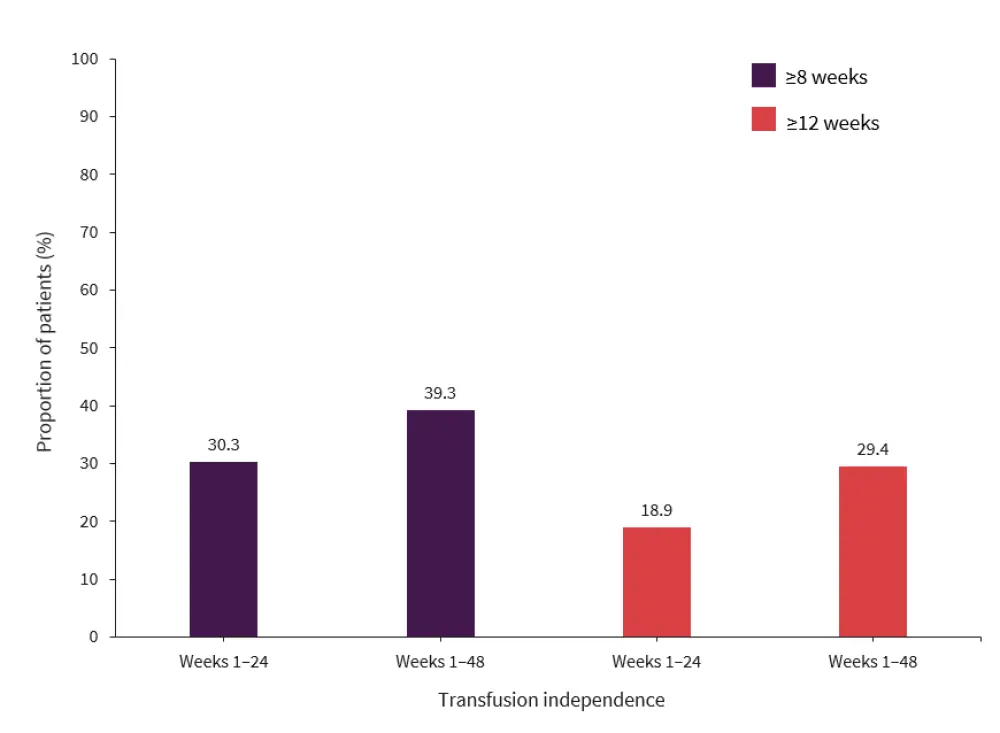
*Data from Lanino, et al.3
Safety
Overall, the safety profile was tolerable:
- Serious adverse events occurred in 17.4% of patients, the most frequent being cardiac events (n = 11), infections (n = 10), COVID-19 pneumonia (n = 4), falls leading to bone fractures (n = 4), and acute kidney injury (n = 1).
- Grade 4 thrombocytopenia and neutropenia occurred in one and eight patients, respectively.
- Advancement to acute myeloid leukemia and death was reported in 5 and 20 patients.
- Treatment discontinuations occurred in 43.4% of patients.
- Main reasons for treatment discontinuation were lack of benefit or loss of response (64.4%), death (14.9%), and consent withdrawal (4.6%).
Conclusion
In the US-based study,1,2 most patients received luspatercept as second-line treatment. ESAs were used as first-line treatment prior to luspatercept initiation in clinical practice. Additionally, in patients with low or moderate TB prior to luspatercept treatment, TB was significantly reduced, a high proportion of patients achieved TI, and over half of patients achieved physician reported mHI-E during Weeks 1–24. The FISiM study3 demonstrated that luspatercept was effective and safe in treating transfusion-dependent anemia in clinical practice, resulting in significantly reduced transfusion burden and achievement of TI. Additionally, baseline TB was identified as a predictive factor for clinical benefit of luspatercept treatment. These findings could help improve clinical management and optimize treatment strategies for patients with MDS-RS with transfusion-dependent anemia.
Overall, both studies showed the clinical benefit of luspatercept in achieving TI, reducing TB, and improving hematological responses, thus supporting its real-world use in clinical practice.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content





