All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Luspatercept for ESA-naïve TD patients with LR-MDS: Interim analysis from the COMMANDS study
Do you know... In which of the following endpoints is treatment with luspatercept likely to improve versus epoetin alfa, based on the results from the COMMANDS trial?
For patients with lower-risk myelodysplastic syndromes (LR-MDS), treatment focuses on the management of anemia and improving quality of life.1 Patients with LR-MDS may require red blood cell (RBC) transfusions to alleviate the symptoms of anemia. Epoetin alfa, an erythropoiesis-stimulating agent (ESA), is often the standard of care treatment for patients with LR-MDS.
However, responses with epoetin alfa are transient, and patients may experience relapse. Luspatercept is an erythroid maturation agent approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in 2020 for the treatment of anemia in adult patients with LR-MDS. In the phase III MEDALIST trial, luspatercept reduced the RBC transfusion burden in patients with LR-MDS; however, no study has compared the efficacy and safety of luspatercept versus epoetin alfa in ESA-naïve patients with LR-MDS.1
The phase III COMMANDS trial (NCT03682536) evaluated luspatercept versus epoetin alfa for the treatment of anemia in transfusion-dependent, ESA-naïve patients with LR-MDS.1 Here we summarize an interim analysis from the COMMANDS trial recently published by Uwe Platzbecker et al.1 in The Lancet. Results from this interim analysis were also presented by Garcia-Manero2 during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, and Della Porta3 at the European Hematology Association (EHA) 2023 Hybrid Congress.
Study design and patient characteristics1–3
The open label, randomized controlled, phase III COMMANDS trial included 142 centers across 26 countries. This interim analysis included the results of efficacy and safety of luspatercept versus epoetin alfa for 300 patients who had either completed 24 weeks of treatment or had discontinued treatment before 24 weeks. The data cutoff date for this analysis was August 31, 2022.
Figure 1. COMMANDS study overview*
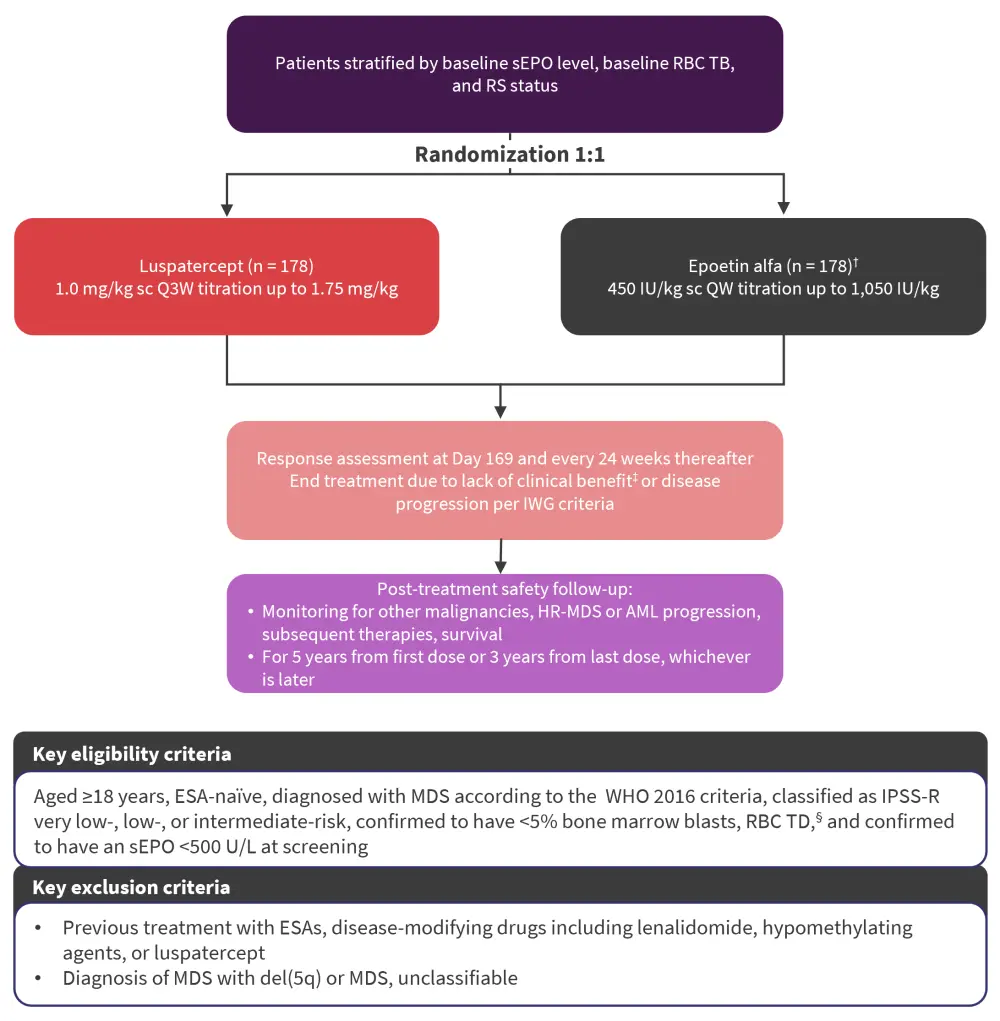
AML, acute myeloid leukemia; ESA, erythropoiesis-stimulating agent; HR, high risk; IPSS-R, revised International Prognostic Scoring System; IWG, International Working Group; MDS, myelodysplastic syndromes; pRBC, packed RBC; QW, once weekly; Q3W, every 3 weeks; RBC, red blood cell; RS, ring sideroblast; sc, subcutaneously; sEPO, endogenous serum erythropoietin concentration; TB, transfusion burden; TD, transfusion-dependent; WHO, World Health Organization.
*Adapted from Garcia-Manero2 and Della Porta.3
†Two patients randomized to epoetin alfa withdrew consent prior to their first dose.
‡Clinical benefit defined as transfusion reduction of ≥2 pRBC units/8 weeks versus baseline.
§2–6 pRBC units/8 weeks for a minimum of 8 weeks immediately before randomization.
- The primary endpoint was RBC transfusion independence (TI) for ≥12 weeks with a concurrent mean hemoglobin increase of ≥1.5 g/dL during Week 1 to Week 24.
- Secondary endpoints analyzed during Week 1 to Week 24 included RBC-TI for 24 weeks, RBC-TI for ≥12 weeks, and hematological improvement-erythroid (HI-E) response ≥8 weeks.
- Exploratory endpoints included subgroup analysis by stratification, baseline characteristics, and the effect of somatic mutations associated with MDS on primary endpoint response.
- Selected ad hoc endpoints included duration of RBC-TI lasting ≥12 weeks with a concurrent mean hemoglobin increase of ≥1.5 g/dL, reduction by ≥50% in RBC units transfused over ≥12 weeks and ≥24 weeks during the entire treatment phase, and a safety analysis at 24 weeks of exposure to both treatments.
A total of 356 patients were randomized to receive either luspatercept or epoetin alfa, of which 301 patients were included in the interim analysis (luspatercept, n = 147; epoetin alfa, n = 154). Baseline patient characteristics are detailed in Table 1. The median treatment duration was 42 weeks (interquartile range, 20–73) in the luspatercept group and 27 weeks (interquartile range, 19–55) in the epoetin alfa group.
Table 1. Baseline patient characteristics*
|
BM, bone marrow; IPSS-R, revised International Prognostic Scoring System; ITT, intention-to-treat; IQR, interquartile range; MDS, myelodysplastic syndromes; MLD, multiple lineage dysplasia; MPN, myeloproliferative neoplasms; RBC, red blood cell; RS, ring sideroblasts; SLD, single lineage dysplasia; T, thrombocytosis; TB, transfusion burden; WHO, World Health Organization. |
||
|
Characteristics, % (unless otherwise specified) |
Luspatercept |
Epoetin alfa (n = 178) |
|---|---|---|
|
Median age (IQR), years |
74 (68–80) |
75 (69–80) |
|
Sex |
|
|
|
Male |
60 |
51 |
|
Female |
40 |
49 |
|
Time since original diagnosis of MDS (range), months† |
8.0 (2.0–28.8) |
5.2 (1.6–18.5) |
|
WHO 2016 classification |
|
|
|
MDS-SLD |
1 |
2 |
|
MDS-MLD |
28 |
26 |
|
MDS-RS-SLD |
1 |
3 |
|
MDS-RS-MLD |
70 |
66 |
|
MDS/MPN-RS-T |
1 |
2 |
|
Missing‡ |
0 |
1 |
|
IPSS-R |
|
|
|
Very low |
9 |
10 |
|
Low |
71 |
74 |
|
Intermediate |
19 |
16 |
|
High |
1§ |
0 |
|
Missing¶ |
1 |
1 |
|
Serum erythropoietin concentration (range), U/L |
78.7 (41.7–185.3) |
85.9 (40.5–177.8) |
|
Serum erythropoietin category, U/L |
|
|
|
≤200 |
79 |
79 |
|
≤100 |
56 |
58 |
|
>100 and ≤200 |
23 |
21 |
|
>200 and <500 |
21 |
21 |
|
Ring sideroblasts‖ |
73 |
72 |
|
Mutated SF3B1‖ |
63 |
58 |
|
RBC-TB (range), units per 8 weeks** |
3 (2–4) |
3 (2–4) |
|
RBC-TB category |
|
|
|
<4 units per 8 weeks |
64 |
61 |
|
2 units per 8 weeks |
45 |
44 |
|
≥4 units per 8 weeks |
36 |
39 |
|
Pretransfusion hemoglobin concentration (range), g/dL |
7.8 (7–8) |
7.8 (7–8) |
|
Hemoglobin category |
|
|
|
≤8 g/dL |
60 |
60 |
|
≥8 g/dl |
40 |
40 |
|
Platelet count (range), 109/L |
230 (155–304) |
235 (140–324) |
Key findings
Primary and second endpoints1–3
In the luspatercept group, 58.5% of patients reached and maintained RBC-TI for ≥12 weeks, with a concurrent mean hemoglobin increase of ≥1.5 g/dL compared with 31.2% of patients in the epoetin alfa group (common risk difference on response rate, 26.6; 95% confidence interval [CI], 15.8−37.4; p < 0.0001; odds ratio [OR], 3.1; 95% CI, 1.9–5.0). Luspatercept was also associated with a better or similar likelihood of achieving RBC-TI for ≥12 weeks with a concurrent mean hemoglobin increase of ≥1.5 g/dL when compared with epoetin alfa for each subgroup analyzed (Figure 2).
Figure 2. Primary endpoint subgroup analysis*
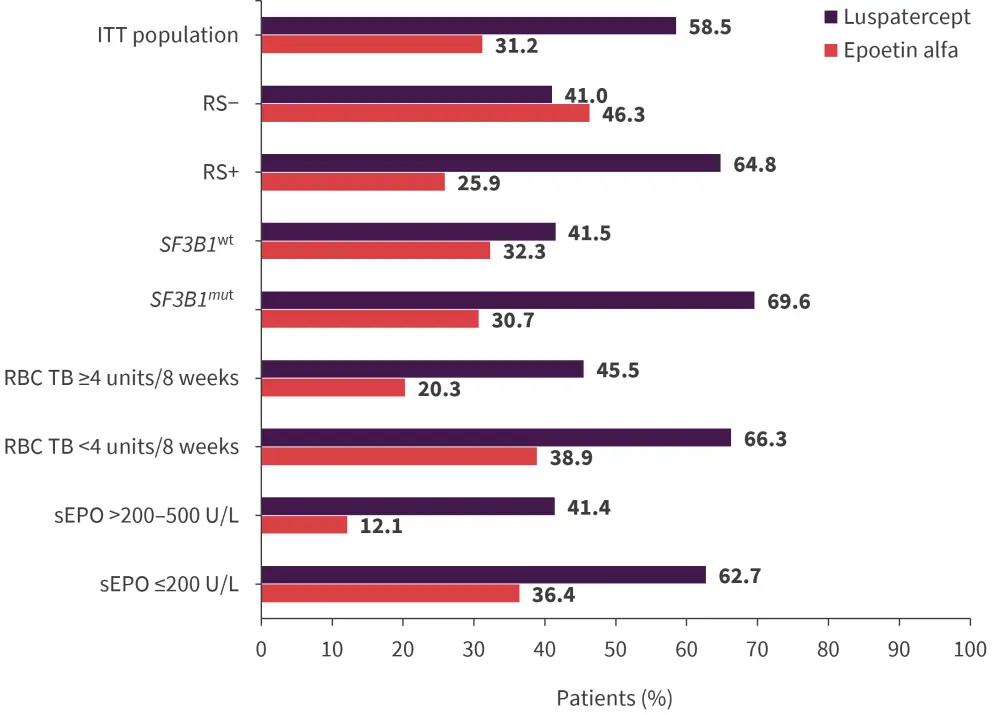
ITT, intention to treat; mut, mutated; RBC, red blood cell; RS, ring sideroblasts; sEPO, serum erythropoietin concentration; TB, transfusion burden; wt, wild type.
*Adapted from Garcia-Manero2 and Della Porta.3
Compared with the epoetin alfa group, more patients in the luspatercept group were RBC-TI for ≥12 weeks during Week 1 to Week 24 (OR, 2.4; 95% CI, 1.5–4.0; p = 0.0002), for 24 weeks during Week 1 to Week 24 (OR, 2.3; 95% CI, 1.4–3.8; p = 0.0006), and had a HI-E response (OR, 2.8; 95% CI, 1.7–4.6; p < 0.0001; Figure 3).1
Figure 3. RBC-TI and HI-E response during Week 1 to Week 24*
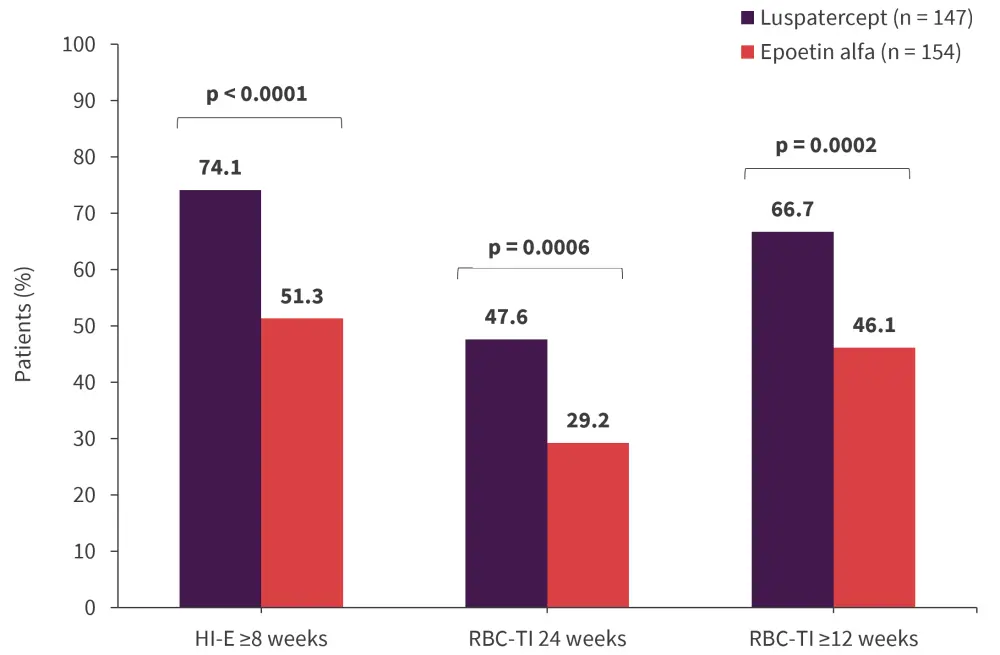
HI-E, hematological improvement-erythroid; RBC, red blood cell; TI, transfusion independence.
*Adapted from Platzbecker, et al.,1 Garcia-Manero,2 and Della Porta.3
In the luspatercept group, 58% of patients were RBC-TI for ≥24 weeks during Week 1 to Week 48 versus 35% of patients in the epoetin alfa group (common risk difference on response rate, 21.8; 95% CI, 10.9–32.8; p < 0.0001).
The median duration of RBC-TI was 127 weeks versus 77 weeks in the luspatercept and epoetin alfa groups, respectively (p = 0.005). This benefit was observed regardless of transfusion burden, SF3B1 mutational status, endogenous serum erythropoietin concentration, and ring sideroblast status (Table 2). Among patients with a HI-E response, the mean time to HI-E was 17.1 days (standard deviation, 29.3) in the luspatercept group versus 27.0 days (standard deviation, 33.9) in the epoetin alfa group.
Table 2. Duration of RBC-TI*
|
CI, confidence interval; HR, hazard ratio; RBC, NE, not evaluable; red blood cell; RS, ring sideroblasts; TB, transfusion burden; TI, transfusion independence. |
|||
|
Median duration (95% CI), weeks |
Luspatercept |
Epoetin alfa |
HR (95% CI) |
|---|---|---|---|
|
Serum erythropoietin category ≤200 U/L |
140.1 (112.7–NE) |
77.0 (41.9–NE) |
0.601 (0.348–1.038) |
|
Serum erythropoietin category >200 U/L–500 U/L |
48.3 (26.3–93.0) |
23.9 (14.9–NE) |
0.624 (0.186–2.092) |
|
RS+ |
120.9 (76.4–NE) |
47.0 (36.6–NE) |
0.626 (0.361–1.085) |
|
RS− |
NE (46.0–NE) |
95.1 (35.3–NE) |
0.492 (0.148–1.638) |
|
TB <4 RBC units/8 weeks |
126.6 (108.3–NE) |
89.7 (41.9–NE) |
0.662 (0.366–1.197) |
|
TB ≥4 RBC |
112.7 (30.0–NE) |
36.6 (24.6–NE) |
0.480 (0.187–1.233) |
|
Mutated SF3B1 |
120.9 (93.0–NE) |
47.0 (36.6–NE) |
0.573 (0.320–1.026) |
|
Non-mutated SF3B1 |
NE (37.0–NE) |
95.1 (35.3–NE) |
0.820 (0.316–2.128) |
Mutational analysis1,3
Baseline mutational data were available for 295 patients included in this analysis. The most commonly mutated genes were SF3B1, TET2, ASXL1, DNMT3A, U2AF1, and SRSF2. Mutation frequencies were comparable between both treatment groups. Patients with mutations in ASXL1, TET2, SF3B1, and SF3B1α (SF3B1 mutation with concomitant mutation of DNM3TA or ASXL1 and/or TET2) were more likely to reach RBC-TI for ≥12 weeks, with a concurrent mean hemoglobin increase of ≥1.5 g/dL when treated with luspatercept versus epoetin alpha (Figure 4).
Figure 4. Mutational subgroup analysis
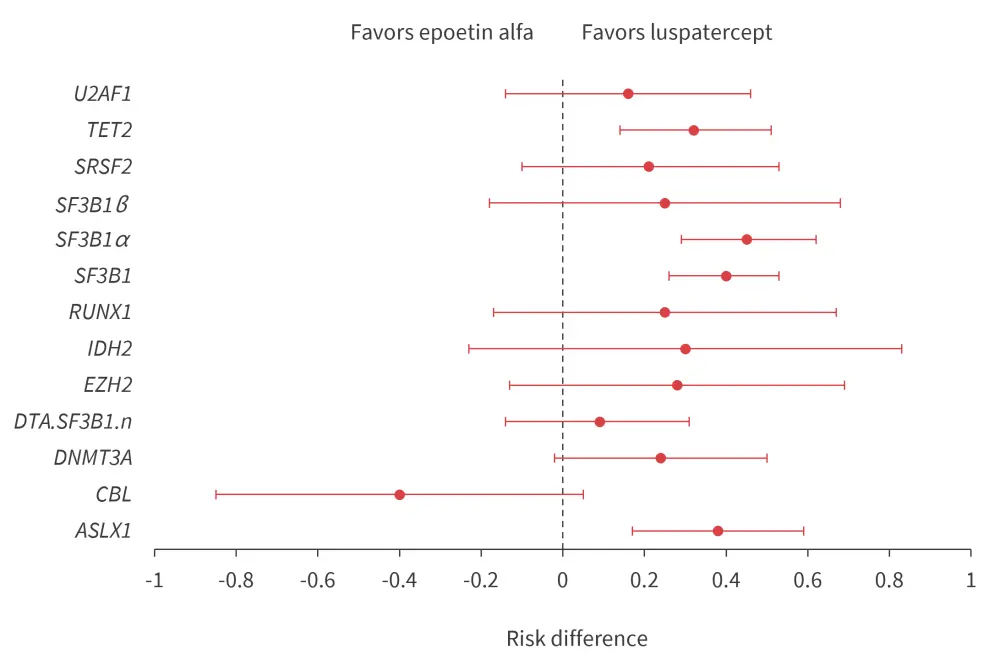
DTA.SF3B1.n, SF3B1 wild-type with concomitant mutations in ASXL1 and/or TET2 or DNMT3A; SF3B1α, SF3B1 mutation with concomitant mutation of DNM3TA or ASXL1 and/or TET2; SF3B1β, mutated SF3B1 with concomitant mutations in BCOR, BCORL1, NRAS, RUNX1, SRSF2 or STAG2.
*Adapted from Platzbecker, et al.1 and Della Porta.3
Safety analysis1–3
In total, 354 patients were included in the safety analysis. In the luspatercept group, 92.1% of patients experienced at least one treatment-emergent adverse event (TEAE) versus 85.2% of patients in the epoetin alfa group (Table 3). TEAEs leading to death occurred in 5% and 7% of patients in the luspatercept and the epoetin alfa groups, respectively. In the luspatercept group, 44% of patients discontinued treatment, with the main reasons for discontinuation including lack of efficacy (15.7%), death (6.2%), adverse events (4.5%), and disease progression (3.9%). In the epoetin alfa group, 60% of patients discontinued treatment, with the main reasons for discontinuation including lack of efficacy (32.4%), death (6.3%), disease progression (4.0%), and adverse events (2.3%).
Table 3. TEAEs observed in both arms*
|
TEAE, treatment-emergent adverse event; TEE, thromboembolic event. |
||||
|
Adverse event, % |
Luspatercept |
Epoetin alfa |
||
|---|---|---|---|---|
|
Any grade |
Grade 3–4 |
Any grade |
Grade 3–4 |
|
|
Hematological related TEAEs |
|
|
|
|
|
Anemia |
9.6 |
7.3 |
9.7 |
6.8 |
|
Thrombocytopenia |
6.2 |
3.9 |
1.7 |
0.6 |
|
Neutropenia |
5.1 |
3.9 |
7.4 |
0.0 |
|
Leukocytopenia |
1.1 |
0.0 |
1.7 |
0.0 |
|
TEAEs of interest |
|
|
|
|
|
Fatigue |
14.6 |
0.6 |
6.8 |
0.6 |
|
Diarrhea |
14.6 |
1.1 |
11.4 |
0.6 |
|
Peripheral edema |
12.9 |
0.0 |
6.8 |
0.0 |
|
Asthenia |
12.4 |
0.0 |
14.2 |
0.6 |
|
Nausea |
11.8 |
0.0 |
7.4 |
0.0 |
|
Dyspnea |
11.8 |
3.9 |
7.4 |
1.1 |
|
TEE |
4.5 |
2.8 |
2.8 |
0.6 |
Conclusion
This interim analysis demonstrated that the primary endpoint of RBC-TI for ≥12 weeks was achieved with luspatercept in a head-to-head study with epoetin alfa in ESA-naïve transfusion-dependent patients with LR-MDS. This benefit was observed across all the subgroups and was independent of the mutational status. The safety profile of luspatercept was consistent with previous reports. Luspatercept could be an alternative to the standard treatment for anemia; however, the results from this study will need to be further evaluated when long-term follow-up data become available.
Your opinion matters
Once there are signs that a TD LR-MDS non-del5q patient has failed ESA, what would be your next approach? A) Evaluate for 2L treatment w luspatercept B) Keep on ESA, given patient has not significantly progressed C) Evaluate for 2L treatment w/ HMAs D) Stop & re-initiate ESA
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



