All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Anemia treatment with luspatercept in patients with lower-risk MDS: results from the PACE-MDS study
Patients with myelodysplastic syndromes (MDS) will often experience anemia, with treatments for lower-risk (LR) MDS focusing on this condition. Luspatercept is a first-in-class erythroid-maturation agent approved by the U.S Food and Drug Administration (FDA) for the treatment of anemia in patients with MDS with ring sideroblasts (MDS-RS) who are transfusion dependent and do not respond to, or are ineligible for, erythropoiesis-stimulating agent treatment. This decision was based on results from the MEDALIST trial (NCT02631070), which has been previously covered on the MDS Hub.
Continuing our editorial theme on supportive care in MDS, we present the key findings from the phase II PACE-MDS trial (NCT01749514/NCT02268383) published in J Clin Oncol In 2022 by Platzbecker et al.1 This study examined the efficacy and safety of luspatercept in patients with both MDS-RS and MDS non-RS, patients who were transfusion dependent, non-transfusion dependent, and previously untreated. This highlights an important unmet need as 70–80% of patients with MDS are non-RS.1
Safety was the primary endpoint of this study, with secondary endpoints including rates of hematologic improvement (HI) erythroid (HI-E), HI neutrophil (HI-N), and HI platelet (HI-P). Biomarker quantitation and mutation data were exploratory endpoints.1
Methods
The phase II, open-label, multicenter PACE-MDS study assessed the safety and efficacy of luspatercept in patients with lower-risk MDS (LR-MDS) including patients with non-RS and non-transfusion dependent patients for up to 5 years. Eligible patients were
- aged ≥18 years;
- had an International Prognostic Scoring System defined Low- or Intermediate-1-risk MDS or non-proliferative chronic myelomonocytic leukemia; and
- had anemia with or without red blood cell (RBC) transfusion support.
For the purpose of this study, non-transfusion-dependent patients were defined as patients who did not receive RBC transfusions within 8 weeks before Cycle 1, Day 1 (C1D1). Low transfusion-burden patients were defined as patients who received <4 RBC units within 8 weeks before C1D1, and high transfusion-burden patients were defined as patients who received ≥4 RBC units within 8 weeks before C1D1.
Results
Patient characteristics
A total of 108 patients were included in the PACE-MDS study, including 44 patients with MDS non-RS (Table 1). The median age of this cohort was 72.5 years (range, 29–90 years). The median time since original diagnosis of MDS was 1.62 months (range, 0.04–13.62 months). The International Prognostic Scoring System risk classification determined that 38.9% of patients were low-risk, while 58.3% and 2.8% were intermediate-1- and intermediate-2-risk, respectively.
Table 1. Patient characteristics*
|
EB-1, excess blasts; EPO, erythropoietin; ESA, erythropoiesis-stimulating agent; HTB, high transfusion burden; LTB, low transfusion burden; MDs, myelodysplastic syndromes; MDS-MLD, MDS with multilineage dysplasia; MDS-RS, MDS with ring sideroblasts; NTD, non-transfusion dependent; RS, ring sideroblast; SF, serum ferritin. |
||||||
|
Characteristic, % |
Total |
RS |
Non-RS |
NTD |
LTB |
HTB |
|---|---|---|---|---|---|---|
|
Sex |
|
|
|
|
|
|
|
Female |
33.3 |
35.5 |
31.8 |
38.2 |
37.9 |
26.7 |
|
Median baseline EPO |
163.1 |
132.3 |
286.1 |
128.9 |
186.8 |
269 |
|
Median baseline SF |
1,100 (42.4–4,438) |
1,227 (83.9–4,438) |
753 (42.4–4,152) |
562.8 (125.3–2,532) |
940.9 (42.4–2,508) |
1,610 (83.9–4,438) |
|
Gene mutations |
|
|
|
|
|
|
|
SF3B1 |
43.5 |
74.2 |
2.3 |
44.1 |
34.5 |
48.9 |
|
SRSF2 |
11.1 |
6.5 |
18.1 |
8.8 |
20.7 |
6.7 |
|
U2AF1 |
3.7 |
1.6 |
6.8 |
2.9 |
6.9 |
2.2 |
|
ZRSR2 |
4.6 |
0 |
11.4 |
8.8 |
0 |
4.4 |
|
WHO subtypes |
|
|
|
|
|
|
|
EB-1 |
11.1 |
9.7 |
11.4 |
5.9 |
0 |
22.2 |
|
MDS-RS |
16.7 |
29 |
0 |
29.4 |
6.9 |
13.3 |
|
MDS-MLD |
22.2 |
1.6 |
52.3 |
20.6 |
31 |
17.8 |
|
MDS-RS-MLD |
33.3 |
58.1 |
0 |
26.5 |
44.8 |
31.1 |
|
Other |
15.7 |
1.6 |
34.1 |
17.6 |
17.2 |
13.3 |
|
Missing |
0.9 |
0 |
2.3 |
0 |
0 |
2.2 |
|
Previous therapy |
|
|
|
|
|
|
|
Lenalidomide |
7.4 |
11.3 |
2.3 |
2.9 |
3.4 |
13.3 |
|
Iron |
29.6 |
37.1 |
18.2 |
2.9 |
17.2 |
57.8 |
|
ESA |
44.4 |
51.6 |
36.4 |
29.4 |
37.9 |
60 |
Response rates
Clinically meaningful responses were observed irrespective of RS, SF3B1-mutation status, or baseline transfusion burden (Figure 1–3). RBC-transfusion independence (RBC-TI) ≥8 weeks was not significantly different between the patients with MDS-RS and MDS-non-RS (p = 0.139). RBC-TI ≥8 weeks was significantly different between patients with low transfusion burden and patients with a high transfusion burden (p < 0.001). While RBC-TI ≥8 weeks was not significant between patients with wild-type SF3B1 and SF3B1 mutations (p = 0.710), a significant difference was seen in patients with non-SF3B1 splicing factor mutations (p = 0.026) and patients with no splicing factor mutations (p = 0.018)
International Working Group HI-E was significantly different between patients with MDS-RS and MDS-non-RS (p = 0.002). Overall, HI-E was not significantly different between transfusion burden groups (p = 0.115). However, HI-E was significantly different between patients with SF3B1 mutations and patients with wild-type SF3B1, patients with non-SF3B1 splicing factor mutations, and patients with no splicing factor mutations (p = 0.031). Overall, HI-N and HI-P were not affected by SF3B1 mutational status or splicing factor mutational status.
Figure 1. Overall response and response rate by RS status*
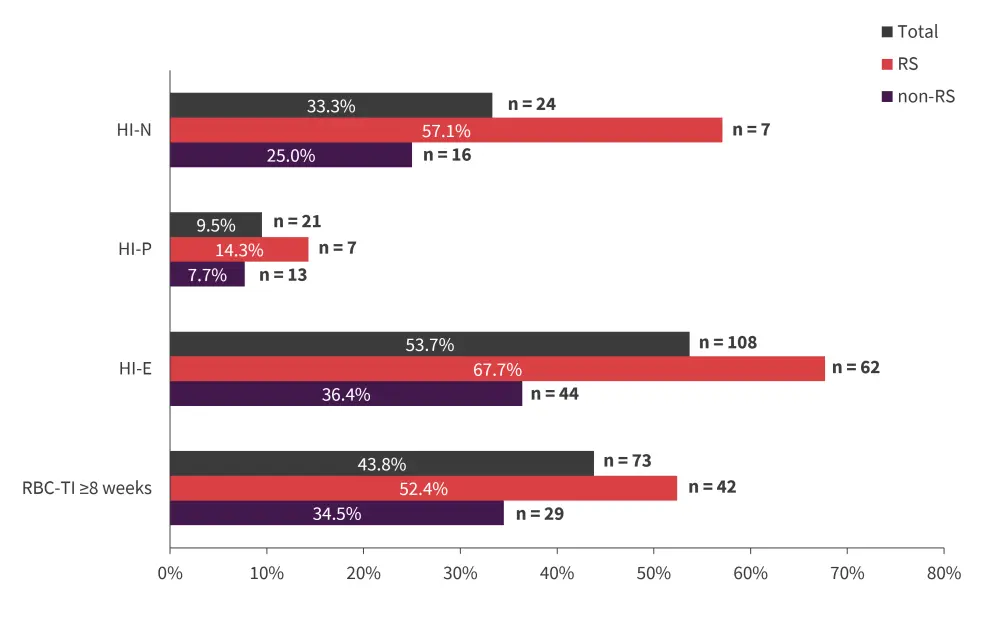
HI-E, hematologic improvement erythroid; HI-N, HI neutrophil; HI-P, HI platelet; RBC-TI, red blood cell transfusion independence; RS, ring sideroblast.
*Data from Platzbecker, et al.1
Figure 2. Response rate by mutation status*
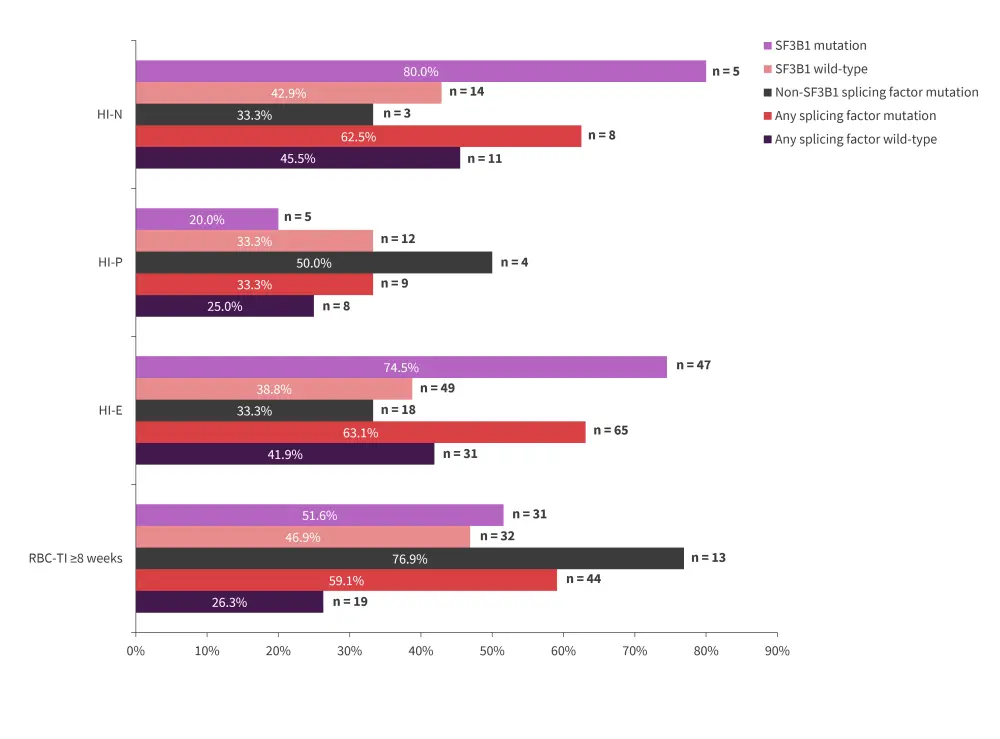
HI-E, hematologic improvement erythroid; HI-N, HI neutrophil; HI-P, HI platelet; RBC-TI, red blood cell transfusion independence.
*Data from Platzbecker, et al.1
Figure 3. Response rate by transfusion burden status*
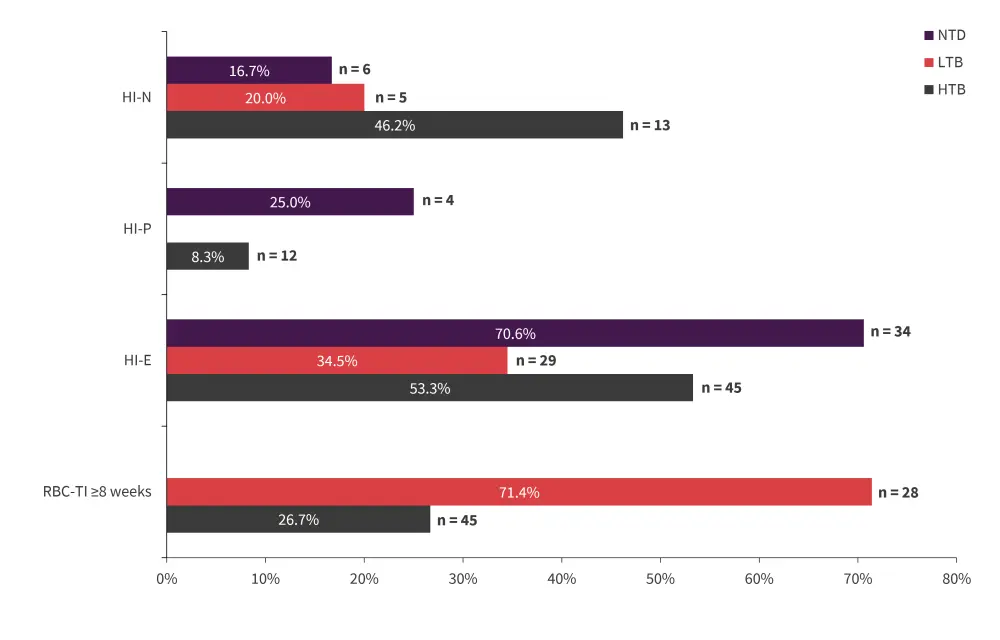
HI-E, hematologic improvement erythroid; HI-N, HI neutrophil; HI-P, HI platelet; HTB, high transfusion burden; LTB, low transfusion burden; NTD, non-transfusion dependent; RBC-TI, red blood cell transfusion independence.
*Data from Platzbecker, et al.1
Safety
Treatment-emergent adverse advent rates for patients with MDS-RS and MDS-non-RS were 48.4% and 36.4%, respectively (p = 0.11; Table 2).
Table 2. TEAEs of any grade occurring in >5% of patients*
|
RS, ring sideroblast; TEAE, treatment-emergent adverse event. |
|||
|
TEAEs, % |
Overall (N = 108) |
RS (n = 62) |
Non-RS (n = 44) |
|---|---|---|---|
|
Any related TEAE |
42.6 |
48.4 |
36.4 |
|
Fatigue |
7.4 |
9.7 |
4.5 |
|
Headache |
7.4 |
4.8 |
11.4 |
|
Hypertension |
6.5 |
6.5 |
6.8 |
|
Arthralgia |
4.6 |
4.8 |
4.5 |
|
Bone pain |
4.6 |
3.2 |
6.8 |
|
Diarrhea |
4.6 |
6.5 |
2.3 |
Biomarkers of hematopoiesis at baseline and during treatment
Analysis of bone marrow in patients with MDS-RS showed a significantly higher baseline ratio of late to early progenitor cells in HI-E responders, increased soluble transferrin receptor concentration, and a lower percentage of proerythroblasts and basophilic erythroblasts. In patients with MDS-non-RS, only lower baseline serum erythropoietin was associated with HI-E (Table 4).
Table 4. Baseline erythropoiesis biomarkers in evaluable HI-E responders versus non-responders by RS status*
|
EPO, erythropoietin; HI-E, hematological improvement erythroid; RS, ring sideroblast. |
|||||||
|
Parameter at |
HI-E response |
RS |
Non-RS |
||||
|---|---|---|---|---|---|---|---|
|
% |
Median (range) |
p value |
% |
Median (range) |
p value |
||
|
Late/early |
Responder |
71.9 |
10.44 (3.22–50.41) |
0.0106 |
42.1 |
3.78 (1.42–7.68) |
0.1074 |
|
Non |
28.1 |
4.48 (0.25–9.90) |
57.9 |
5.41 (2.09–33.49 |
|||
|
Proerythroblasts |
Responder |
71.9 |
5.5 (1.04–18.07) |
0.0236 |
42.1 |
13.9 (7.91–23.55) |
0.0758 |
|
Non |
28.1 |
10.9 (4.75–57.46) |
57.9 |
7.2 (0.99–16.87) |
|||
|
Basophilic |
Responder |
71.9 |
2.4 (0.73–8.75) |
0.0019 |
42.1 |
6 (2.45–13.33) |
0.1074 |
|
Non |
28.1 |
4.5 (3.43–19.22) |
57.9 |
3.4 (0.03–14.97) |
|||
|
Baseline EPO, IU/L |
Responder |
71.9 |
128.2 (22.3–1,995) |
0.0445 |
35.29 |
69.6 (15.4–424.4) |
0.0077 |
|
Non |
28.1 |
122 (43.40–607.7) |
64.5 |
623.3 (98.4–1,571) |
|||
|
Baseline soluble |
Responder |
74.2 |
73.2 (30.70–181.1) |
0.0445 |
38.9 |
28.3 (23.7–136.1) |
0.8563 |
|
Non |
25.8 |
51.8 (28.5–86.4) |
61.1 |
34.1 (8.8–249.7) |
|||
Conclusion
The overall safety of luspatercept exposure for ≥2 years in patients with LR-MDS was confirmed by the PACE-MDS trial, with no new safety signals emerging; these results were confirmed across the different MDS subtypes, including MDS-non-RS.
The differences between the patients with MDS-RS population and patients with MDS-non-RS population were notable, with HI-E response associated with lower baseline erythropoietin levels in patients with MDS-non-RS but not in patients with MDS-RS. Significant changes from baseline to end-of-treatment late-stage erythropoiesis measures were observed in all patients that achieved a long-term HI-E, in line with the mechanism of luspatercept.
The PACE-MDS study was underpowered due to the small sample size; however, the trends that emerged require confirmation. Clinical responses were observed across the different patient subgroups and data supported the mechanism of luspatercept in LR-MDS as RS status-independent.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



