All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Safety and efficacy of enasidenib in IDH2-mutated myelodysplastic syndrome
Enasidenib is an isocitrate dehydrogenase-2 (IDH2) inhibitor approved by the U.S. Food and Drug Administration in 2017 for treating adult patients with relapsed/refractory acute myeloid leukemia (AML) with an IDH2 mutation as confirmed by an FDA-approved test.1 The incidence of mutations in IDH2 in patients with myelodysplastic syndromes (MDS) is between 4–12%, and the incidence is shown to be higher in high-risk disease settings and in cases of hypomethylating agent (HMA) failure.2
Enasidenib is currently under evaluation for the treatment of high-risk and relapsed/refractory MDS—available data in this patient population is limited at present.2 Findings from two previous phase II trials investigating enasidenib monotherapy in relapsed/refractory IDH2-mutated MDS demonstrated encouraging outcomes; further information can be found here.
At the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition, Lionel Adés2 presented the preliminary findings from the phase II IDEAL trial (NCT03744390) investigating the safety and efficacy of enasidenib in patients with MDS. We summarize the key findings below.
Methods
This study included patients based on the following key inclusion criteria:
- Age ≥18 years
- MDS based on the World Health Organization (WHO) criteria including non-proliferative AML up to 29% of bone marrow blasts
- Harboring a IDH2 mutation
- Normal organ function
The primary endpoint was overall hematologic response, including complete response (CR), partial response (PR), and stable disease with hematologic improvement (HI) according to the International Working Group (IWG) 2006 criteria.
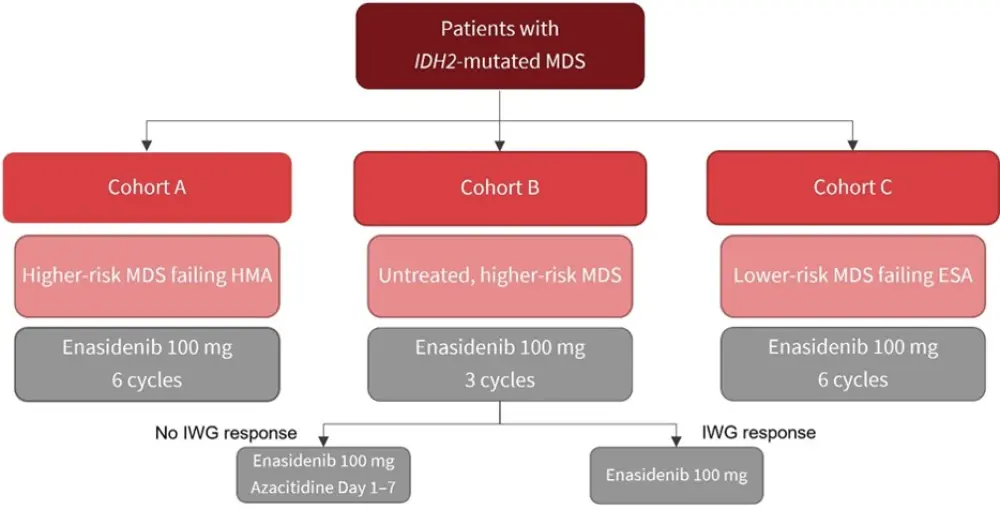
Patients were stratified into three disease cohorts (Figure 1):
- Cohort A included patients with higher-risk MDS (defined as intermediate-2 or high risk according to the International Prognostic Scoring System [IPSS]) having failed HMA. Responders after six cycles were allowed to continue enasidenib.
- Cohort B included untreated patients with higher-risk MDS without life threatening cytopenia (defined as an absolute neutrophil count <0.5 × 109/L or any recent severe infections and/or platelets <30 × 109/L and any bleeding symptom). After three cycles, azacitidine was added to enasidenib for non-responders.
- Cohort C included patients with lower-risk MDS that failed on erythropoiesis-stimulating agents (ESA).
Figure 1. Study design*
ESA, erythropoiesis-stimulating agent; HMA, hypomethylating agent; IWG, International Working Group.
*Adapted from Adés, et al.2
Each cycle was 28 days and repeated until disease progression, relapse, leukemic transformation, unacceptable toxicity, or death.
Results
Baseline characteristics
The analysis included 26 evaluable patients with a median age of 75.5 years; 65% of patients were male. The variant allele frequency of IDH2 mutation was 36%. Table 1 summarizes the patient characteristics by cohort.
Table 1. Patient characteristics*
|
IPSS, International Prognostic Scoring System; VAF, variant allele frequency. |
|||
|
Characteristic |
Cohort A |
Cohort B |
Cohort C |
|---|---|---|---|
|
Median age, years |
77 |
78 |
67 |
|
Sex, % Male Female |
45 55 |
78 22 |
83 17 |
|
IPSS category Low Intermediate-1 Intermediate-2 High |
— 1 7 3 |
— 1 6 2 |
1 5 — — |
|
IDH2 VAF, % |
30 |
42 |
44 |
Safety
At data cutoff date, ten patients were still on treatment. The main reasons for treatment discontinuation were disease progression (23.1%), treatment failure (7.7%), adverse events (AEs; 7.7%), and death (3.8%). Of 26 patients evaluated, eight experienced one Grade ≥3/4 AE, and six experienced two or more Grade ≥3/4 AEs. A differentiation syndrome, which resolved without an issue, was observed in three patients. Other most common Grade 3/4 AEs were nausea/diarrhea (n = 4) and thrombocytopenia (n = 5).
Efficacy
At a median follow-up of 8.6 months, 11 patients (42%) achieved overall best response in the intent-to-treat population; CR was achieved in six patients (23%). Among responders, two patients (n = 1 in cohorts B and C) lost their response after 80 days of treatment. In cohort B, three patients did not respond to enasidenib and were given the combination of enasidenib and azacitidine; two then achieved a response. Figure 2 compares ORR and CR in study cohorts.
Figure 2. ORR by cohort
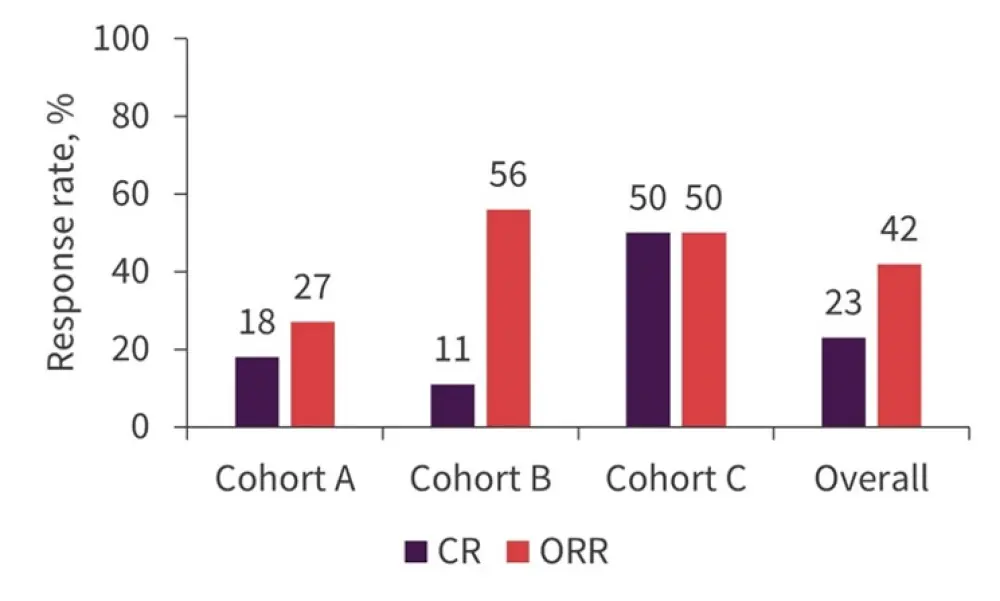
CR, complete response; ORR, overall response rate.
*Adapted from Adés, et al.2
The median overall survival (OS) was 17.3 months; six patients died (n = 4 in cohort A, n = 1 each in cohorts B and C).
- The 6-month death rate was 8.2%, suggesting a low early death rate.
- 1-year OS was 55.4%, 100%, and 80% in cohorts A, B, and C respectively.
- Four patients in cohorts A and B (n = 2 in each) progressed to AML and the risk of leukemic transformation at 1 year was 19.3%. No transformation was observed in cohort C.
Conclusion
This study demonstrated encouraging response rates in patients with IDH2-mutated MDS and requires further observation in a larger study population with a longer follow-up. Safety analysis did not indicate a limiting toxicity. The association of minimal residual disease and clonal architecture with response are being investigated in correlative studies.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

