All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Molecular profiling in myelodysplastic syndromes (MDS) has improved understanding of the pathophysiology involved in progression of the disease, leading to disease subtypes and risk classification. With growing evidence of an array of acquired genetic and signaling aberrations in hematopoietic progenitors, research into novel agents for MDS has started to incorporate targeted therapies. While not a curative option, such therapies may increase quality of life and improve survival outcomes when used as frontline or maintenance treatment, particularly when combined with chemotherapy regimens or approved hypomethylating agents (HMAs).1
This article is the first as part of an editorial theme on the therapeutic landscape for MDS and summarizes the current status of targeted agents for MDS, either in combination approaches or alone. We provide a summary on current clinical data evaluating safety and efficacy of the most researched agents targeting molecular pathways associated with worse prognosis.
Targeted therapies
- Risk-based therapeutic approaches are mostly guided according to the revised International Prognostic Scoring System (IPSS-R).2
- Risk is classified according to age and cytogenetic risk groups; however, there is no inclusion of testing for genetic mutations of interest (e.g., FLT3, IDH2).
- With evidence of poor prognosis associated with certain genetic mutations, targeted therapies have primarily been of interest for patients classified as having high-risk MDS. The primary goal is to overcome low response rates associated with the use of the HMAs azacitidine (aza) or decitabine, which are the two agents currently approved by the U.S. Food and Drug Administration (FDA).
- Targeted therapies with extensive clinical data include FLT3-, IDH1-, and BCL2-inhibitors, and TP53 reactivators.
- Targets for approved and potential therapies are summarized in Figure 1.
Figure 1. Overview of genetic and signaling targets for approved and experimental therapies*†
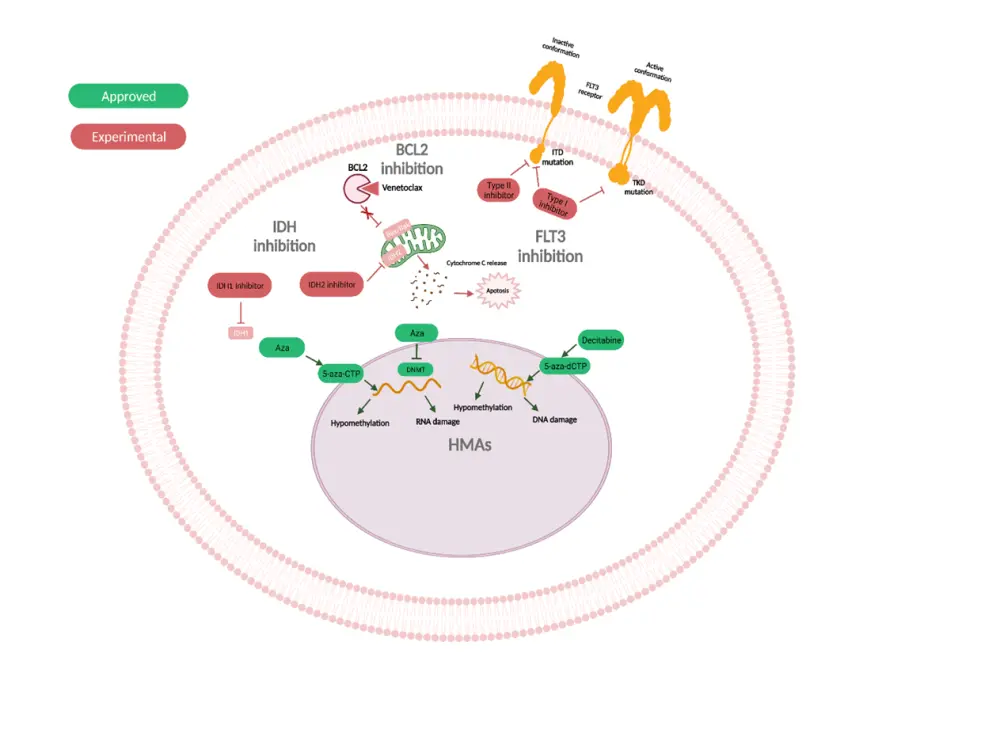
5-aza-CTP, 5-aza-2'-cytidine triphosphate; 5-aza-dCTP, 5-aza-2'-deoxycytidine triphosphate; Aza, azacitidine; BCL2, B-cell lymphoma 2; FLT3, Fms-related receptor tyrosine kinase 3; HMAs, hypomethylating agents; IDH, isocitrate dehydrogenase; TKD, tyrosine kinase domain.
*Adapted from Pagliuca et al.1
†Created with BioRender.com
Venetoclax
Venetoclax (Ven) is a promising novel agent with a primary mode of action to inhibit the regulator protein B-cell lymphoma 2 (BCL2), which in turn increases apoptosis of lymphocytic cells. It has been evaluated extensively in patients with acute myeloid leukemia (AML) and is FDA-approved in combination with HMAs or low-intensity chemotherapy for patients ineligible for intensive chemotherapy. More recent research has investigated the use of venetoclax in combination with HMAs, particularly azacitidine, for patients with high-risk MDS.
- A retrospective analysis of 20 patients, summarized on the MDS Hub, reported an overall response rate (ORR) of 75% with a combination of either decitabine or azacitidine + venetoclax. However, an unfavorable safety profile was demonstrated, with high rates of toxicities and myelosuppression.3
- Additionally, results from the phase Ib M15-531 study (NCT02942290) showed a favorable ORR of 77% with venetoclax + azacitidine in 57 patients with treatment-naïve, high-risk MDS.4
- Median overall survival (OS) in this study was not reached.
- The M15-531 study supported breakthrough therapy designation from the FDA for a combination of venetoclax + azacitidine in patients with treatment-naïve MDS. You can read a full summary of this designation on our hub here.
Hematologic toxicities with venetoclax plus azacitidine
- Hematologic toxicities associated with venetoclax + azacitidine relate mostly to cytopenia, and the most effective strategies to manage adverse events (AEs) from this combination is of growing interest.
- At the 26th Congress of the European Hematology Association (EHA2021), Uwe Platzbecker summarized data from two safety expansion cohorts from the M15-531 study, investigating dose modifications of azacitidine + venetoclax.5
- Safety Cohort I (SE1) had the venetoclax or azacitidine dose initially reduced while safety Cohort II (SE2) had a treatment schedule consisting of stepwise dose reductions of azacitidine initially, followed by a shortened venetoclax duration.
- No significant difference was reported for hematologic treatment-emergent AEs (TEAEs) between the safety cohorts.
- There was no effect on response rates reported in either cohort.
- You can find our interview with Uwe Platzbecker from EHA2021 summarizing this study below.
What hematologic toxicities occur after venetoclax + azacitidine in MDS and how can they be managed?
Venetoclax plus chemotherapy
- A recent phase II trial investigated venetoclax plus intensive chemotherapy with cladribine, idarubicin, and cytarabine in 50 patients with newly diagnosed AML or high-risk MDS. (NCT02115295)6
- The combination demonstrated durable responses with a high composite complete response (CR) rate (47/50; 94% for all patients including MDS), ORR (94%), and event-free survival (68%) reported. However, the small number of patients with MDS included (n = 4) limits the conclusions.
- The most frequent Grade >3 AEs were febrile neutropenia (84% of patients), infection (12%) and elevated ALT (12%).
Clinical trial data on venetoclax combinations are summarized in Table 1 below.
Table 1. Clinical trials assessing venetoclax combinations*
|
HMA, hypomethylating agents; N/A, not applicable; ORR, overall response rate;. |
||||
|
Agents |
NCT number |
Disease type |
Study/phase |
ORR |
|---|---|---|---|---|
|
HMA + azacitidine3 |
N/A |
High-risk |
Retrospective analysis |
75% |
|
Venetoclax + azacitidine4 |
High-risk treatment naïve |
Ib |
77% |
|
|
Venetoclax + intensive chemotherapy6,† |
Newly diagnosed or high-risk |
II |
94% |
|
FLT3 inhibitors
When assessing the impact of disease genotype on prognosis and response to treatment, mutations of fms-related receptor tyrosine kinase 3 (FLT3) have emerged as a rare (< 5%) but key driver of worsened prognosis. FLT3 internal tandem duplication and other FLT3 mutations equates with high-risk classification and is associated with greater rates of relapse following hematopoietic stem cell transplantation (HSCT).1
Midostaurin
- Most research into the efficacy of FLT3 inhibitors has focused on AML. Midostaurin has emerged as a potential therapy following HSCT, demonstrating favorable relapse-free survival in the phase II RADIUS trial, summarized on our hub.
- For MDS, combination approaches with midostaurin appear promising, albeit producing modest results. A phase I/II trial assessed the efficacy of midostaurin in combination with azacitidine for untreated patients who were not able or refused to receive standard therapy, and patients with refractory or relapsed (R/R) AML. The investigators reported a median OS of 22 weeks (95% CI, 15−29 weeks), and an ORR of 26%.7
- However, only a small number of patients with MDS (n = 3) were included.
Quizartinib
- Quizartinib is another FLT3 inhibitor that has been investigated in AML. No data are available for efficacy or safety for MDS yet, nevertheless, clinical trials are currently enrolling to investigate a combination of this inhibitor with decitabine and venetoclax in patients with high-risk MDS (Table 2).
Gilteritinib
- In the phase II study assessing venetoclax plus intensive chemotherapy with cladribine, idarubicin, and cytarabine, researchers also included a cohort with FLT3-mutated AML/MDS that were given a FLT3 inhibitor.6
- Eight out of nine patients received gilteritinib, a second-generation inhibitor of both FLT3 and AXL, while one patient received midostaurin.
- An ORR of 89% (8/9 patients) was reported, seven of whom had a CR, while one patient had a CR with incomplete blood count recovery.
- In terms of safety, the addition of FLT3 inhibitors appeared to prolong myelosuppression.
-
- One patient died during induction from bacteremia and sepsis before count recovery, and this patient was receiving concomitant FLT3 inhibitor.
- Three patients died in CR, two of whom received a FLT3 inhibitor. These deaths were determined to be from infectious complications while cytopenic mid-course, however were not considered to be treatment related.6
- Gilteritinib and midostaurin are now being compared in a recruiting trial in combination with induction and consolidation therapy followed by 1-year maintenance in patients with FLT3-mutated MDS (NCT04027309).
Table 2. Clinical trials assessing FLT3 inhibitor agent combinations*
|
AML, acute myeloid leukemia; MDS, myelodysplastic syndromes; N/A, not applicable; NCT, National Clinical Trial; ORR, overall response rate. |
|||||
|
Agents |
NCT number |
Phase |
Disease type |
Stage |
ORR |
|---|---|---|---|---|---|
|
Midostaurin + azacitidine7 |
I/II |
Relapsed MDS and AML |
Active |
26% |
|
|
Quizartinib + decitabine + venetoclax |
I/II |
Untreated/relapsed AML |
Recruiting |
— |
|
|
Gilteritinib or midostaurin + venetoclax + intensive chemotherapy6 |
II |
AML and High-risk MDS |
Active |
89% |
|
|
Gilteritinib or midostaurin + induction and consolidation therapy |
I |
Newly diagnosed AML or MDS |
Recruiting |
N/A |
|
IDH inhibitors
Mutations of isocitrate hydrogenase (IDH) are also rare in MDS (approximately 5−10%), however, they are another class of genetic abnormalities that infer poor prognosis and are associated with leukemic transformation.1 The IDH1 inhibitor ivosidenib and IDH2 inhibitor enasidenib have demonstrated potential efficacy in HMA-naïve or refractory patients and appear to be well tolerated.
Ivosidenib
- Ivosidenib is currently approved by the FDA for use in AML, however, not in MDS.
- When investigating its efficacy in MDS, a phase I dose escalation study demonstrated an ORR of 91.7% in patients with R/R MDS, albeit in a small cohort of patients (n = 12)8
- Ivosidenib will also be investigated in a future trial for patients with IDH1-mutated MDS, either alone or in combination with other targeted agents such as venetoclax (Table 3).
Enasidenib
- Enasidenib has been assessed in a phase II trial:
- The investigators reported an ORR of 67% in 18 patients, including HMA-naïve MDS (n = 6) who received a combination of enasidenib + azacitidine, and patients with HMA-refractory MDS (n = 12), who received enasidenib alone.9
- All HMA-naïve patients (n = 6) who received enasidenib plus azacitidine responded to therapy (two patients with CR and four patients with marrow CR).
- The rate of AEs of any grade was 68% (17/25 patients) while Grade 3−4 AEs were reported in 44% (11/25) of patients.
- Notably, possible differentiation syndrome was reported in three patients on Days 31, 38, and 42 of treatment, and one patient was determined to have progressed to AML.
- Enasidenib more recently demonstrated promising efficacy in a phase II trial when used either alone (ORR, 43% [n = 9/21]) for HMA-refractory MDS or in combination with azacitidine in untreated MDS (ORR, 84% [n = 21/25]). The investigators also reported that treatment was well tolerated10:
- Common treatment-related Grade 3−4 AEs in the enasidenib + azacitidine arm were neutropenia (64%), thrombocytopenia (28%), and anemia (8%); these occurred in 10%, 0%, and 5%, in the enasidenib arm, respectively.
- Grade 3−4 infections occurred in 32% of patients receiving enasidenib + azacytidine, and 14% receiving enasidenib alone.
- Enasidenib will be further investigated as a prophylactic consolidation treatment for patients with IDH2-mutated MDS, chronic myelomonocytic leukemia (CMML) and AML, who are in remission after allogeneic stem cell transplantation (allo-SCT) salvage or who have relapsed (Table 3).
Table 3. Clinical trials assessing IDH1/2 agents alone or in combination*
|
HMA, hypomethylating agent; IDH1, isocitrate dehydrogenase; IDH2, isocitrate dehydrogenase 2; MDS, myelodysplastic syndromes; NCT, National Clinical Trial; ORR, overall response rate. |
|||||
|
Agent(s) |
NCT number |
Phase |
MDS disease type |
Trial stage |
ORR |
|---|---|---|---|---|---|
|
Ivosidenib8 |
I |
IDH1 |
Active, Recruiting |
91.7% |
|
|
Ivosidenib |
II |
IDH1 |
Recruiting |
— |
|
|
Ivosidenib + venetoclax |
I/II |
IDH1 |
Recruiting |
— |
|
|
Enasidenib9 |
I/II |
IDH2 |
Active, not recruiting |
67% |
|
|
Enasidenib10 |
II |
IDH2, HMA refractory |
Completed |
43% |
|
|
Enasidienib + azacitidine10 |
II |
IDH2, untreated |
Completed |
84% |
|
|
Enasidenib |
II |
IDH2 |
Recruiting |
— |
|
TP53
Mutations in TP53 gene are associated with worsened survival outcomes and high progression in both AML and MDS.1 One potential therapy is eprenetapopt (APR-246), which is a p53 reactivator recently demonstrating promising efficacy and safety in two phase II trials when combined with azacitidine. You can find these trials summarized on our hub here.
- The first trial (NCT03072043) revealed an ORR of 73% (29/40) for patients with MDS, and a CR rate of 50%.11
- The most commonly reported Grade ≥3 AEs were febrile neutropenia (33%), leukopenia (29%), and neutropenia (29%).
- The second trial, GFM-APR246 (NCT03588078), reported an ORR of 62% in 34 patients with MDS, and a CR rate of 47%.12
- The most commonly observed AEs were febrile neutropenia (37%) and neurologic events (40%), and Grade ≥3 AEs were only observed in three patients.
- A phase III trial for patients with TP53-mutated MDS was initiated following these results; however, on December 28, 2020, the trial sponsors announced that the primary endpoint for CR had not been met. You can read more about this here.
Conclusion
Genotyping has opened up a plethora of new therapeutic opportunities for patients with MDS, who have been historically limited to chemotherapy, HMAs, or HSCT. Targeted therapies present potential for improved survival in high-risk patients who are ineligible for intensive chemotherapy, relapse following HSCT, or who are refractory to HMAs. They appear to be most effective when combined with existing treatments, particularly azacitidine, demonstrating efficacy in the frontline and more moderately in the relapse setting. However, limiting toxicity is an important aspect for certain novel combinations such as venetoclax + azacitidine.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Uwe Platzbecker
Uwe Platzbecker