All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Luspatercept reduces red blood cell transfusion burden on patients with very low to intermediate risk MDS without negative impact on health-related quality of life
Myelodysplastic syndromes (MDS) are a heterogenous group of clonal hematopoietic neoplasms characterized by ineffective hematopoiesis, progressive cytopenias, and the risk of progression to acute myeloid leukemia.1 Around 90% of patients with MDS experience anemia, often leading to fatigue, cardiac morbidity, and reduced health-related quality of life (HRQoL).1 Treatments for patients diagnosed with low-risk MDS are primarily aimed at alleviating anemia; however, patients who are refractory to erythropoiesis-stimulating agents (ESA) can only receive red blood cell (RBC) transfusions, providing transient and short-term relief of anemia-related symptoms.1 Long-term dependency on transfusions often leads to poor prognosis, causing complications such as hepatic and cardiac organ failure due to iron overload.1 The phase 3 MEDALIST trial (NCT02631070) compared the treatment of luspatercept, a first-in-class erythroid maturation agent and best supportive care (BSC) against a placebo + BSC in the reduction of transfusion burden and HRQoL.
Study design1
This double blind, placebo controlled, randomized phase 3 trial had a 2:1 ratio of luspatercept (1–1.75 mg/kg) or the placebo administered subcutaneously every 3 weeks for 24 weeks. A total of 229 patients were included in the study, 149 in the luspatercept + BSC group and 76 in the placebo + BSC group. The primary endpoint was patient transfusion independence of 8 weeks or greater and the secondary endpoint was transfusion independence of 12 weeks or greater, both assessed from Weeks 1 to 24 and 1 to 48. HRQoL was evaluated as a secondary endpoint.
Patient selection
Patients aged 18 or over with a documented diagnosis of very low-risk, low-risk, or intermediate-risk MDS with ring sideroblasts were included in the study. Each patient must also have been receiving regular RBC transfusions and be either refractory, intolerant, or ineligible for ESA treatment. Patients were excluded if they experienced a consecutive 56-day period that was RBC transfusion free during the 16 weeks before randomization. Patient demographics and clinical characteristics at baseline are shown in Table 1.
Table 1. Patient demographics and clinical characteristics at baseline of HRQoL-evaluable population*
|
BSC, best supportive care; ESA, erythropoiesis-stimulating agent; IPSS-R, Revised International Prognostic Scoring System; RBCT, red blood cell transfusion; SD, standard deviation. |
|||
|
Characteristic, n (%) |
Luspatercept + BSC |
Placebo + BSC |
Total |
|---|---|---|---|
|
Mean age (SD), years |
70.5 (8.7) |
70.7 (10.9) |
70.6 (9.4) |
|
Age group, years |
|||
|
≤64 |
28 (18.8) |
16 (21.1) |
44 (19.6) |
|
65–74 |
70 (47.0) |
29 (38.2) |
99 (44.0) |
|
≥75 |
51 (34.2) |
31 (40.8) |
82 (36.4) |
|
Sex |
|||
|
Male |
93 (62.4) |
50 (65.8) |
143 (63.6) |
|
Race |
|||
|
White |
105 (70.5) |
51 (67.1) |
156 (69.3) |
|
Black |
1 (0.7) |
0 (0.0) |
1 (0.4) |
|
Not collected |
42 (28.2) |
24 (31.6) |
66 (29.3) |
|
Other |
1 (0.7) |
1 (1.3) |
2 (0.9) |
|
IPSS-R risk |
|||
|
Very low or low |
123 (82.6) |
63 (82.9) |
186 (82.7) |
|
Intermediate |
25 (16.8) |
13 (17.1) |
38 (16.9) |
|
Missing |
1 (0.7) |
0 (0.0) |
1 (0.4) |
|
Prior ESA use |
|||
|
Yes |
144 (96.6) |
70 (92.1) |
214 (95.1) |
|
Transfusion burden |
|||
|
<4 RBCT units/8 weeks |
44 (29.5) |
20 (26.3) |
64 (28.4) |
|
4–5 RBCT units/8 weeks |
40 (26.8) |
23 (30.3) |
63 (28.0) |
|
≥6 RBCT units/8 weeks |
65 (43.6) |
33 (43.4) |
98 (43.6) |
HRQoL assessments
HRQoL for each patient was assessed using the European Organisation for Research and Treatment of Cancer’s Core Quality of Life Questionnaire (EORTC QLQ-C30). The domains of interest on the EORTC QLQ-C30 were global health status, physical functioning, emotional functioning, fatigue, and dyspnea; all were considered the most clinically relevant to MDS. Further domains that were considered exploratory included pain, insomnia, social functioning, and nausea/vomiting. The EORTC QLQ-C30 had scores ranging from 0–100, with higher scores in the quality of life and functioning domains representing better patient quality of life and higher scores in all other domains representing a worse quality of life.
Results
The baseline scores from the EORTC QLQ-C30 were similar between both treatment groups. In all domains, there was no clinically significant difference in the mean change from baseline values for both the luspatercept and placebo group. The impact of treatment-related side effects was also comparable between the groups. Transfusion dependence showed the only meaningful difference, with a greater proportion of patients treated with luspatercept reporting improvements in daily life due to its lower impact (39% vs 22% in the luspatercept vs placebo group, respectively).
In contrast, 12% of patients in the luspatercept group reported transfusion dependence having more of an impact compared with 22% of patients in the placebo group. These results are shown in Figure 1.
Figure 1. Percentage of patients reporting a higher or lower impact on transfusion dependence across both treatment groups*
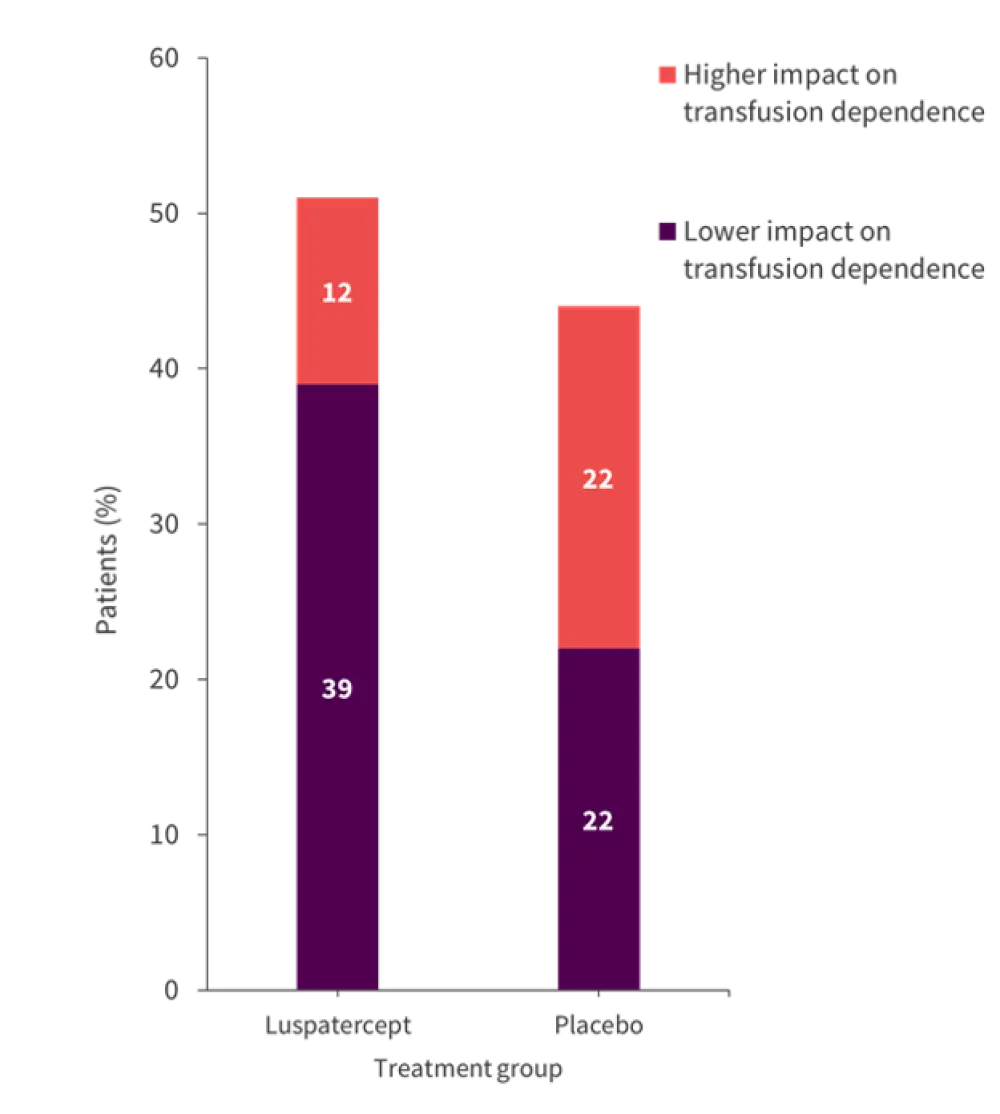
*Data from Oliva, et al.1
Conclusion
Luspatercept + BSC was found to reduce RBC transfusion burden with no clinically meaningful change in HRQoL thresholds. Baselines were similar to those observed in patients with recurrent or metastatic cancer, demonstrating no negative impact from luspatercept treatment. These results have implications for patients who are transfusion dependant, refractory to, intolerant, or ineligible for treatment with ESAs.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

