All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Novel treatment strategies in patients with high-risk and low-risk MDS
This article summarizes key data from three presentations on novel treatments in myelodysplastic syndromes (MDS) from the 64th American Society of Hematology (ASH) Annual Meeting and Exposition. Amer Zeidan1 presented results from the STIMULUS-MDS1 phase II study of sabatolimab, Uwe Platzbecker2 presented phase II study results of imetelstat in low-risk MDS, and Guillermo Garcia-Manero3 presented the outcomes from the phase I/II trial of canakinumab in low-risk MDS.
Sabatolimab in high-risk MDS1
Study design
STIMULUS-MDS1 (NCT03946670) is an ongoing phase II study of sabatolimab that enrolled 127 adult treatment-naïve patients with intermediate-, high-, or very high-risk MDS (as defined by the revised International Prognostic Scoring System). All patients received hypomethylating agents (HMAs) and were randomized 1:1 to also receive either sabatolimab or placebo. The HMA given (azacitidine or decitabine) was decided at the investigator’s discretion, with 89% of patients receiving azacitidine. The primary endpoint of this study was the rate of complete remission (CR) and progression-free survival (PFS). And secondary endpoints included response rates, overall survival (OS), duration of response, and safety.
Results
The median follow-up of the study was 24 months.
The rates of the most common hematologic Grade ≥3 adverse events (AEs) are summarized in Figure 1. The most common non-hematologic all-grade AEs were constipation and diarrhea.
Figure 1. Most common Grade ≥3 adverse events*
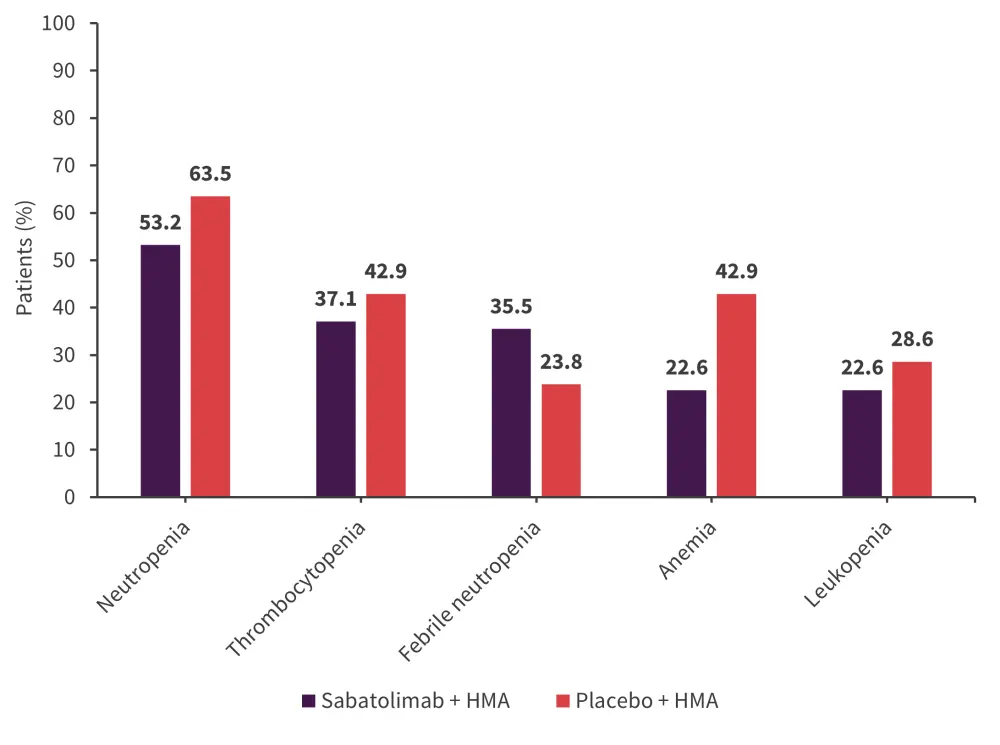
HMA, hypomethylating agent.
*Adapted from Zeidan.1
Treatment discontinuation was 78.5% and 82.3% in the sabatolimab + HMA and placebo + HMA groups, respectively.
The overall response rates are shown in Figure 2. The mean duration of CR was 18 months and 9.2 months in the sabatolimab + HMA and placebo + HMA groups, respectively.
Figure 2. Overall response rate*
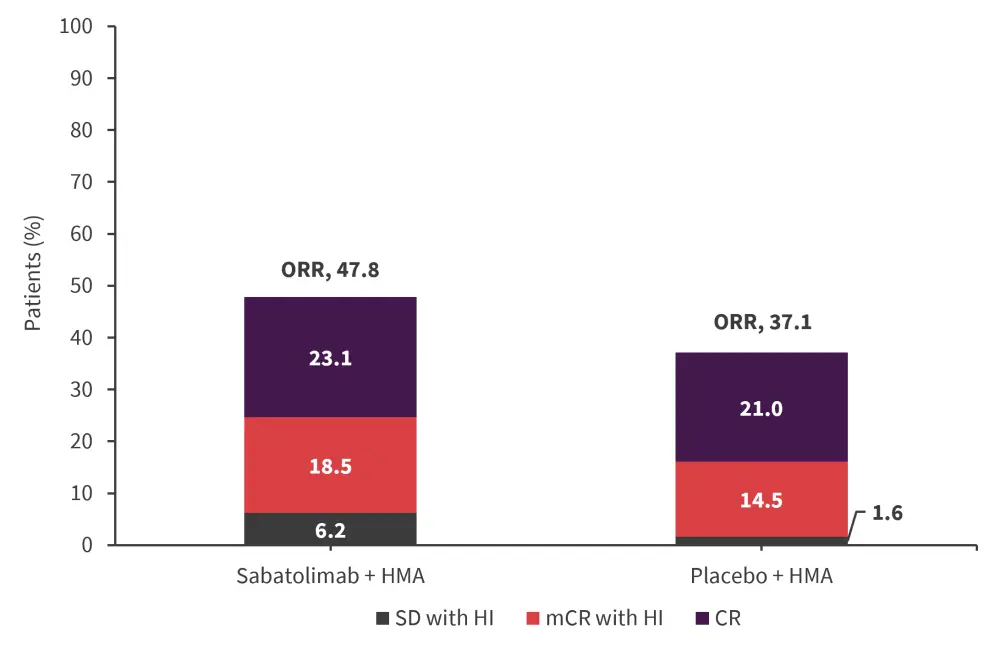
CR, complete remission; HI, hematologic improvement; HMA, hypomethylating agent; mCR, marrow CR; ORR, overall response rate; SD, stable disease.
*Adapted from Zeidan.1
The median overall survival was 19 months and 18 months in the sabatolimab + HMA and placebo + HMA groups, respectively.
In exploratory subgroups of PFS, survival was higher in the sabatolimab + HMA group compared with the placebo + HMA group for patients with a lower Molecular International Scoring System score (23.7 months vs 8.77 months, respectively) and also in patients with <10% blasts (11.3 months vs 8.3 months, respectively).
Presenter’s conclusion
This study shows that sabatolimab + HMA can be used safely in patients with high-risk MDS. Although significant improvements in CR were not seen, exploratory analyses suggest sabatolimab may be more effective in patients with a lower disease burden. There is currently an ongoing phase III trial (STIMULUS-MDS2) which will provide further data on the safety and efficacy of sabatolimab.
Imetelstat in low-risk MDS2
Study design
IMerge is an ongoing phase II/III study (NCT02598661) of imetelstat that enrolled 57 patients with lower-risk MDS. The primary endpoint of this study was ≥8-week red blood cell transfusion independence (TI). Secondary endpoints included safety, 24-week TI rate, MDS response, OS, PFS, and time to progression to acute myeloid leukemia.
Results
- Overall, 38 patients met the target population criteria (having a non-del(5q) mutation and were lenalidomide/HMA-naïve). Of these 38 patients, 11 patients achieved a TI of ≥1 year. In addition, there was a longer duration of TI seen in the target population compared with all patients.
- The mean duration of treatment for patients with TI ≥1 year was 126.1 weeks, with a median of 27 treatment cycles.
- The safety profile was similar between patients with TI ≥1 year and the target population. The most common adverse events were thrombocytopenia (occurring in 63.6% and 60.5% of patients with TI ≥1 year and the target population, respectively) and neutropenia (occurring in 54.5% and 55.3% of patients with TI ≥1 year and the target population, respectively).
- The median OS and PFS are shown in Figure 3 (median follow-up, 51.5 months).
- None of the TI ≥1-year responders progressed to acute myeloid leukemia.
Figure 3. Survival outcomes*
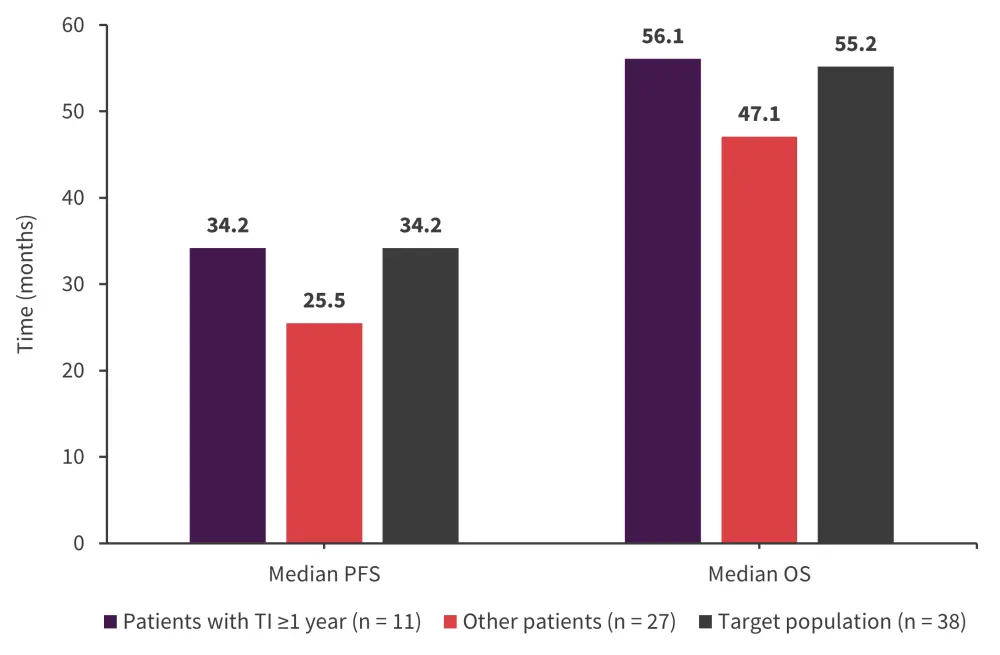
OS, overall survival; PFS, progression-free survival; TI, transfusion independence.
*Adapted from Platzbecker.2
Presenter’s conclusion
The safety profile of imetelstat in this study was similar to previous reports, in addition to providing a durable TI especially in non-del(5q) lenalidomide/HMA-naïve patients. Enrolment in the phase III part of this study is complete, with top-line results expected in January 2023. The phase III study will compare the efficacy of imetelstat with the placebo.
Canakinumab in low-risk MDS3
Study design
This is an ongoing phase II study (NCT04239157) of canakinumab, a monoclonal antibody targeting interleukin-1 beta, in patients with low-risk MDS. The primary objective of this study was the safety and clinical activity of canakinumab, as defined by the International Working Group 2006 response criteria.
Results
- Among the 25 patients enrolled in the study, the most common AEs were anemia and neutropenia, each of which occurred in 11 patients. There were no severe adverse events.
- There was an overall response rate of 16%, with two patients achieving TI, and the median OS for all patients was 16.3 months.
- Single-cell RNA sequencing, revealed that:4
- The two patients with hematologic improvement were found to only have one mutation each, with one patient having a TET2 mutation, and the other having a DNMT3A mutation.
- Patients with progression were found to have multiple mutations.
- In one patient with hematologic improvement, there was increased differentiation in their hematopoietic stem cells post-canakinumab treatment.
- Canakinumab was found to have no effect in patients with an SF3B1 mutation as interleukin-1 beta signaling is not disrupted by this mutation.
Presenter’s conclusion
This study has shown that canakinumab can be used safely in patients with lower-risk MDS. Further studies in patients with earlier-stage MDS and clonal cytopenias with unknown significance will provide further data on the efficacy and safety of canakinumab.
Key takeaway
These results demonstrate that these novel therapies have acceptable safety and efficacy for use in patients with MDS. Sabatolimab was found to have a favorable safety profile and was well tolerated, with similar rates of adverse events to placebo-treated patients. Further data are to be provided by the ongoing STIMULUS-MDS2 trial. Imetelstat provided the best efficacy in patients who were lenalidomide/HMA-naïve with a non-del(5q) mutation. Results from the phase III study will provide further efficacy data. The study of canakinumab showed it can be safely used in patients with lower-risk MDS, although single-cell RNA sequencing suggests it may have greater efficacy in patients with certain mutations and in patients with fewer total mutations.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

