All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Real-world data on MDS with ring sideroblasts after ESA failure
Myelodysplastic syndromes (MDS) represent a group of clonal stem cell disorders. Of the patients who experience MDS with ring sideroblasts (MDS-RS), a subcategory with dysregulated iron metabolism, 90% do so because of an underlying mutation in the SF3B1 gene. Patients with MDS-RS are recognized as low risk for MDS-related sequelae, such as acute myeloid leukemia (AML), and have a good prognosis regarding long-term survival. However, patients with MDS-RS experience particular treatment challenges, such as chronic anemia and red blood cell (RBC) transfusion-dependence, amongst other morbidities.1
Treatments for MDS-RS include erythropoiesis-stimulating agents (ESAs) in the first line, and lenalidomide and hypomethylating agents (HMAs), such as azacitidine, in the second line. However, primary treatment failure and transient responses are common. For these patients, the activin-IIB ligand trap luspatercept has been shown to have clinical benefit in phase II and III trials, eliminating transfusion-dependence in nearly half of patients with MDS-RS after ESA failure.1
Here, we report on the findings of a large cohort study by Sophie Park and colleagues, recently published in Leukaemia Research, exploring the clinical outcomes of patients with MDS-RS after the failure of ESAs.1 They aimed to characterize the outcomes of these patients further and guide the future use of novel agents, such as luspatercept.
Study design
This is an international, multi-center, retrospective cohort study.
Data from a previously published analysis on 1,698 patients with non-del(5q) lower-risk MDS and ESA treatment failure were used.2 Out of these patients, 528 patients with MDS-RS (as per the World Health Organization [WHO] 2008 criteria) were selected for this analysis.
Patients were classified into cohorts of second-line treatment after primary ESA failure:
- Those receiving only HMA, lenalidomide, or RBC transfusion (recognized as best supportive care)
Transfusion burden was classified as:
- Low: 3–7 RBC concentrates in 16 weeks and 2–3 in 8 weeks
- High: ≥ 8 RBC concentrates in 16 weeks and ≥ 4 in 8 weeks
Data analysis
- The outcome of second-line treatment with HMA and lenalidomide vs best supportive care
- Duration of ESA response on AML progression
- Overall survival (OS) from ESA failure
- Outcomes of MDS-RS vs MDS non-RS from ESA treatment initiation and after ESA failure
Results
Patient characteristics are summarized in Table 1. A total of 322 patients (61%) responded to first-line ESA, and presented a median response duration of 22 months in MDS-RS vs 19 months in MDS non-RS patients (p = 0.94).
Compared to responders, short (< 12 months) and no response to ESA in MDS-RS were associated with:
- Younger age (71 years vs 74.8 years, p = 0.0004)
- Higher IPSS-R (84% vs 70%, respectively, p = 0.0002)
- And higher transfusion burden before ESA onset (36% vs 16%, p = 0.0001)
The study follows 160 patients who received subsequent treatments after ESA: in Figure 1, the number of patients responding or not to each line of therapy is represented.
Table 1. Patient characteristics1
|
ESA, erythropoiesis-stimulating agent; HMA, hypomethylating agent; IPSS-R, Revised International Prognostic Scoring System; IQR, interquartile range; LEN, lenalidomide; TX, treatment line after ESA. |
|
|
Variable |
Patients (N = 528) |
|---|---|
|
Median age at onset of ESA, years (IQR) |
74.0 (67–80) |
|
Male, n (%) |
309 (58.5) |
|
IPSS-R at onset of ESA, n (%) |
|
|
Very low |
101 (22.3) |
|
Low |
318 (70.2) |
|
Intermediate |
34 (7.5) |
|
Transfusion burden, n (%) |
|
|
No |
227 (51.5) |
|
Low |
96 (21.8) |
|
High |
118 (26.8) |
|
Median duration of ESA response, months (IQR) |
22.1 (11.0–40.9) |
|
Second treatment after ESA (TX2), n (%) |
N = 160 |
|
HMA |
70 (43.7) |
|
LEN |
90 (56.2) |
|
Median TX2 duration, months (IQR) |
6.7 (4.2–13.4) |
|
Third treatment after ESA (TX3), n (%) |
N = 101 |
|
HMA |
15 (15) |
|
LEN |
86 (85) |
|
Median TX3 duration, months (IQR) |
4.5 (3.9–6.3) |
|
Fourth treatment after ESA (TX4), n (%) |
N = 8 |
|
HMA |
6 (75) |
|
LEN |
2 (25) |
|
Median TX4 duration, months (IQR) |
5.3 (1.5–6.9) |
|
Fifth treatment after ESA (TX5), n (%) |
N = 3 |
|
HMA |
3 (100) |
|
Median TX5 duration, months (IQR) |
6.60 (0.10–48.10) |
Figure 1. Responders and non-responders to first-line treatment with ESA and subsequent treatments1
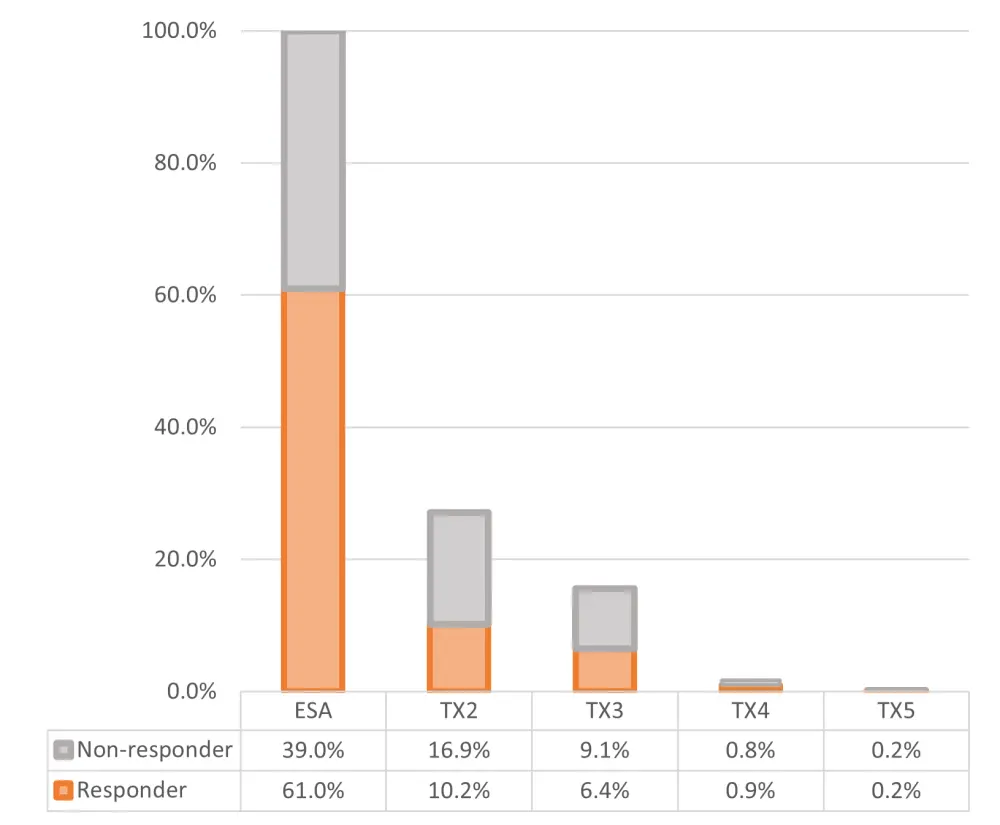
ESA, erythropoiesis-stimulating agent; TX, treatment line after ESA.
With a median follow-up of 45 months:
- 45% of MDS-RS relapses after response to ESA treatment occurred within the first 12 months
- Only treatment with HMA in the second line was associated with an increased risk of progression to AML (HR, 3.3; 95% CI, 1.1–10.4; p = 0.04)
- Table 2 summarizes additional details on patients receiving a second line of treatment and their response
Table 2. Patients with MDS-RS receiving second-line treatment1
|
AZA, azacitidine; ESA, erythropoiesis-stimulating agent; HMA, hypomethylating agent; IPSS-R, Revised International Prognostic Scoring System; IQR, interquartile range; LEN, lenalidomide; MPN-RS, myelodysplastic syndromes with ring sideroblasts; TX2, second-line treatment after ESA failure. |
||
|
TX2 |
||
|---|---|---|
|
HMA (AZA) |
LEN (± ESA) |
|
|
Number of patients |
70 |
90 |
|
Median age (IQR) |
71 (66–78) |
74 (67–77) |
|
IPSS-R at ESA failure, % |
||
|
Very low |
8.5 |
5.5 |
|
Low |
69.5 |
54.5 |
|
Intermediate |
22 |
40 |
|
Response rate (%) |
28.8 |
45.4 |
- The 5-year incidence of AML
- From ESA onset: 5.3% for MDS-RS vs 9.3% MDS non-RS (p = 0.02)
- After ESA failure: 16.6% for MDS-RS vs 13.0% for MDS non-RS (p = 0.57)
- The 5-year OS data is presented in Table 3
Table 3. Five-year OS for MDS-RS and MDS non-RS at ESA onset and after ESA failure
|
CI, confidence interval; ESA, erythropoiesis-stimulating agent; MDS, myelodysplastic syndromes; OS, overall survival; RS, ring sideroblasts. |
|||
|
MDS-RS, % (95% CI) |
MDS non-RS, % (95% CI) |
p value |
|
|---|---|---|---|
|
5-year OS from ESA onset |
65.5 (60–70) |
58.4 (55–61) |
0.0007 |
|
5-year OS after ESA failure |
48.5 (38.0–58.3) |
46.0 (41.9–50.0) |
0.38 |
Conclusion
The study identifies that MDS-RS does not have a statistically significant lower median response rate to ESAs than MDS non-RS. It demonstrated that patients with MDS-RS had improved OS and lower 5-year AML incidence than those with lower-risk MDS without sideroblasts. Some patients within the study received second-line treatment, and these patients receiving lenalidomide or an HMA did not experience a significant increase in OS relative to best supportive care. Patients treated with HMAs in the second line were at an increased risk of progression to AML.
The study establishes that in the real-world setting, HMAs and lenalidomide do not bring a treatment advantage to patients on second-line treatment for MDS-RS with short (< 12 months) or no response to ESA. The authors identify a higher risk group, within which earlier definitive treatment with allogeneic stem cell transplantation or treatment with novel agents such as luspatercept should be considered. Further research is needed to confirm any potential treatment advantage with these modalities.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

