All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Magrolimab plus azacitidine in patients with higher-risk MDS: updated results from a phase Ib study (5F9005)
Magrolimab, a first-in-class anti-CD47 monoclonal antibody, was approved for the treatment of patients with newly diagnosed myelodysplastic neoplasms (MDS) by the U.S. Food and Drug Administration (FDA) on September 15, 2020. This approval was based on preliminary results of a phase Ib study (5F9005, NCT03248479) investigating magrolimab in patients with intermediate to very high-risk MDS who were not eligible for intensive chemotherapy. These results were previously reported by the MDS Hub. Here, we summarize updated results of this phase Ib trial published by Sallman et al.1 in Journal of Clinical Oncology.
Study design and patient characteristics
This was an open-label, single-arm, multicenter trial evaluating the safety and tolerability of magrolimab in adult patients with untreated MDS according to the World Health Organization (WHO) classification and the revised International Prognostic Scoring System (IPSS-R).
The primary endpoints were treatment-emergent adverse events (TEAEs), serious adverse events (AEs) according to the Common Terminology Criteria for AEs Version 4.03, and efficacy measured by investigator-assessed rate of complete remission (CR) by International Working Group 2006 MDS response criteria.
Treatment schedule
- Priming magrolimab dose of 1 mg/kg given intravenously on Days 1 and 4, then 15 mg/kg on Day 8 followed by dose-escalation up to 30 mg/kg on Days 11, 15, and 22 (Figure 1).
- On Days 1–7 of each 28-day cycle, patients were administered azacitidine 75 mg/m2 intravenously or subcutaneously.
- Maintenance dose of 30 mg/kg once weekly or every two weeks.
Figure 1. Dosing schedule*
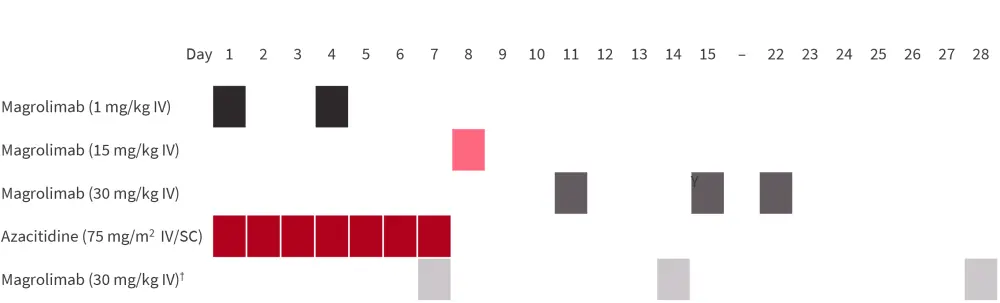
IV, intravenously; SC, subcutaneously.
*Adapted from Sallman, et al.1
†Magrolimab 30 mg/kg once weekly in the initial expansion cohort or maintenance dose interval increased to once daily every 2 weeks beginning Cycle 3 Day 1.
Patient characteristics
In total, 95 patients were enrolled with a median age of 69 years (range, 28–91 years); 69.5% had an Eastern Cooperative Oncology Group (ECOG) Performance Status of 1 or 2, 62.1% had poorer-risk cytogenetics, 27.4% had complex cytogenetics, and 22.1% had therapy-related MDS. The proportion of patients who were intermediate-, high-, or very high-risk measured by IPSS-R was 27.4%, 51.6%, and 21.1%, respectively. Moreover, 26.3% of patients had TP53 mutations and the median exposure to treatment was six cycles (range, 1–27 cycles).
Results
Safety
- The most common TEAEs included constipation, thrombocytopenia, and anemia, with the most common Grade 3 or 4 TEAEs being anemia, neutropenia, and thrombocytopenia (Figure 2).
- Serious AEs included febrile neutropenia, pneumonia, anemia, bacteremia, pyrexia, and infusion-related reactions, reported in 24.2%, 9.5%, 8.4%, 6.3%, 5.3%, and 5.3% of patients, respectively.
- In terms of change in hemoglobin, the median change from baseline to first assessment post-dose was −0.7 g/dL (range, −3.1 to 2.4 g/dL).
- The median maximum drop in hemoglobin was −1.1 g/dL and −0.5 g/dL between the first and second and the second and third magrolimab doses, respectively.
Figure 2. Most common TEAEs in ≥10% of patients, irrespective of causality*
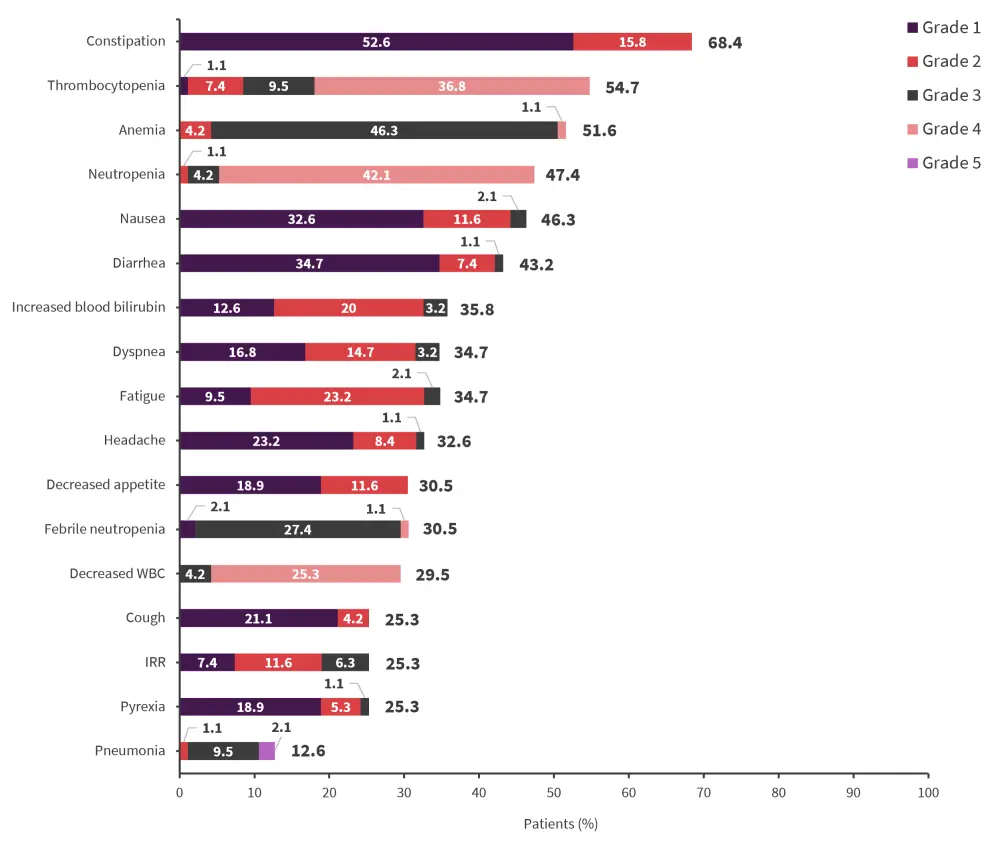
IRR, infusion-related reaction; TEAE, treatment-emergent adverse event, WBC, white blood cell.
*Adapted from Sallman, et al.1
Magrolimab and azacitidine treatment was delayed due to TEAEs in 52.6% and 49.5% of patients, respectively. Treatment discontinuation due to TEAEs occurred in 10.5% of patients and 2.1% of patients died at 60 days; most deaths were due to progressive disease (20%) and AEs (8.4%) during the TEAE assessment period.
Efficacy
Overall response rate was 74.7%, with a median time to first response of 1.9 months (range, 0.7–10.9 months). In total, CR was achieved by 32.6% of patients and the median time to CR was 3.7 months (range, 1.7–7.2 months). In patients with TP53 mutations, CR was achieved by 40%, with a median overall survival of 16.3 months. Efficacy outcomes in patients with and without detectable TP53 mutation at baseline are shown in Table 1.
Table 1. Efficacy outcomes*
|
CI, confidence interval; CR, complete remission; HI, hematologic improvement; mCR, marrow complete remission; MDS, myelodysplastic neoplasms; mut, mutated; NR, not reached; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial remission; RBC, red blood cell; SD, stable disease; TI, transfusion independence; wt, wild-type. *Adapted from Sallman, et al.1 |
|||
|
Efficacy outcome, % (unless stated otherwise) |
All patients |
TP53-wt MDS |
TP53-mut MDS |
|---|---|---|---|
|
ORR‡ |
74.7 |
78.7 |
68.0 |
|
CR (95% CI) |
32.6 (23.4–43.0) |
31.1 (19.9–44.3) |
40.0(21.1–61.3) |
|
mCR |
31.6 |
37.7 |
20.0 |
|
PR |
0.0 |
0.0 |
0.0 |
|
SD with HI |
10.5 |
9.8 |
8.0 |
|
Median duration of CR (95% CI), months |
11.1 (7.6–13.4) |
12.9 (8.0–NR) |
7.6 (3.1–13.4) |
|
Median duration of ORR (95% CI), months |
9.8 (8.8–12.9) |
9.8 (8.5–18.5) |
9.2 (5.0–12.2) |
|
mCR with HI |
16.8 |
19.7 |
12.0 |
|
Any HI |
58.9 |
60.7 |
56.0 |
|
Converted to RBC TI§ |
35.1 |
26.1 |
46.2 |
|
Median PFS (95% CI), months |
11.6 (9.0–14.0) |
11.8 (8.8–16.6) |
11.0 (6.3–12.8) |
|
Median OS (95% CI), months |
NR (16.3–NR) |
NR (21.3–NR) |
16.3 (10.8–NR) |
Conclusion
This phase Ib trial demonstrated the favorable safety profile of magrolimab + azacitidine in patients with untreated high-risk MDS, including those with TP53 mutations. The magrolimab + azacitidine combination also showed promising efficacy in this population. Further safety and efficacy evaluation of the combination of magrolimab with azacitidine is currently ongoing in the phase III, double-blind, placebo-controlled, randomized controlled trial ENHANCE (NCT04313881).
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

