All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
CELMoDs for the treatment of non-Hodgkin lymphoma
Featured:
Do you know... In the phase I/II CC-99282-NHL-001 study, a cohort of patients with heavily pre-treated DLBCL received golcadomide + rituximab. Which of the following treatment subgroups had the highest overall response rate?
During the Lymphoma Hub Steering Committee Meeting, Gilles Salles, Memorial Sloan Kettering Cancer Center, New York, US, chaired a discussion on cereblon E3 ligase modulators (CELMoDs) for the treatment of non-Hodgkin lymphoma. This discussion also featured Catherine Thieblemont, Francesc Bosch, Grzegorz Nowakowski, Marek Trněný, Michael Dickinson, Miles Prince, Sonali Smith, and Ulrich Jäger.
CELMoDs for the treatment of non-Hodgkin lymphoma
CELMoDs for the treatment of non-Hodgkin lymphoma
Salles began by presenting an overview of CELMoDs, including their mechanism of action (Figure 1), and introducing iberdomide and golcadomide as novel agents under investigation for the treatment of lymphomas. Salles discussed early efficacy and safety insights from the phase I/II CC-220-NHL-001 trial (NCT04464798) of iberdomide in patients with relapsed/refractory (R/R) lymphomas and key efficacy and safety outcomes from the phase I/II CC-99282-NHL-001 trial (NCT03930953) of golcadomide in patients with R/R follicular lymphoma (FL) and R/R diffuse large B-cell lymphoma (DLBCL) (Figure 2 and Figure 3, respectively).
Figure 1. CELMoDs: Mechanism of action
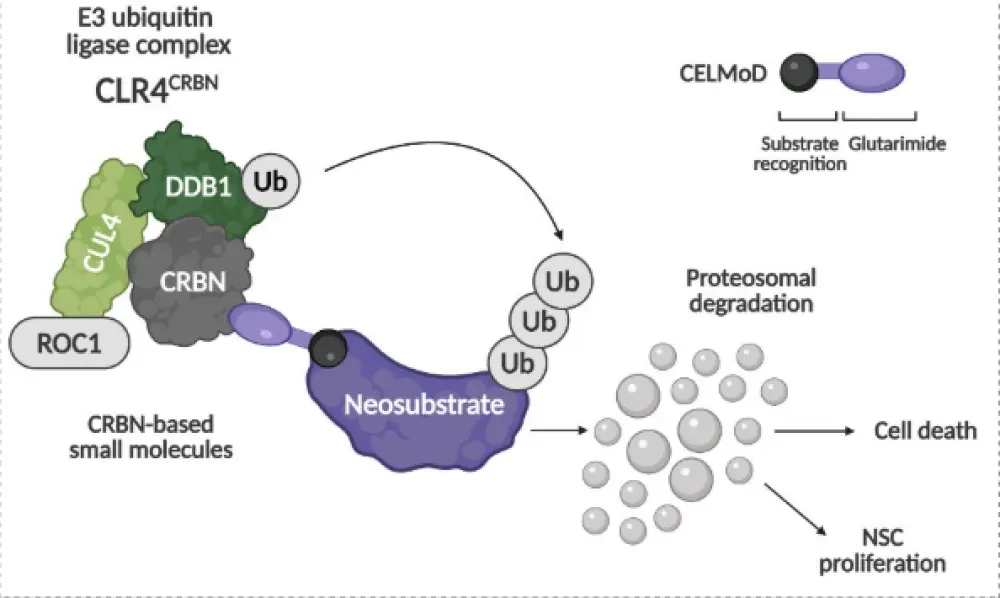
Figure 2. Preliminary efficacy of iberdomide in R/R lymphomas: Results from the CC-220-NHL-001 trial*
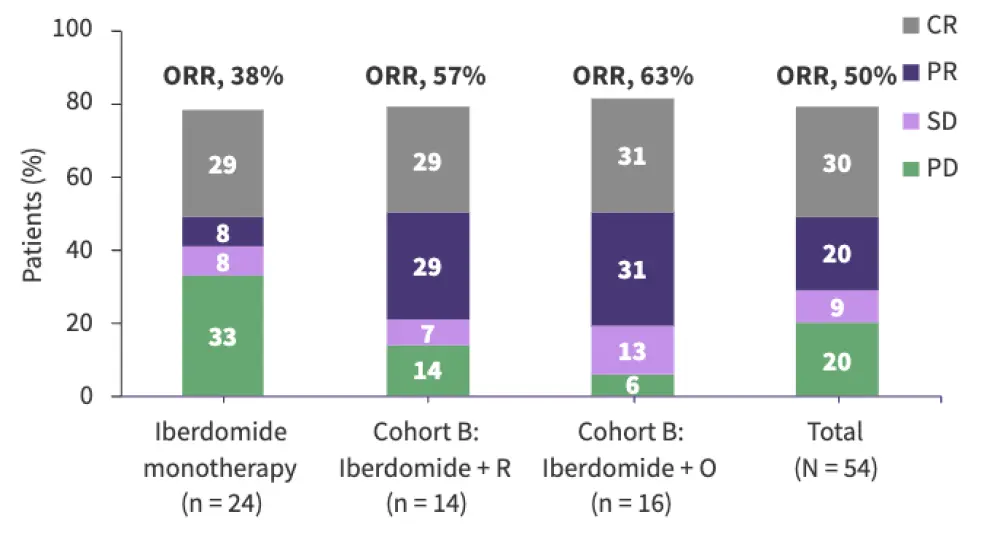
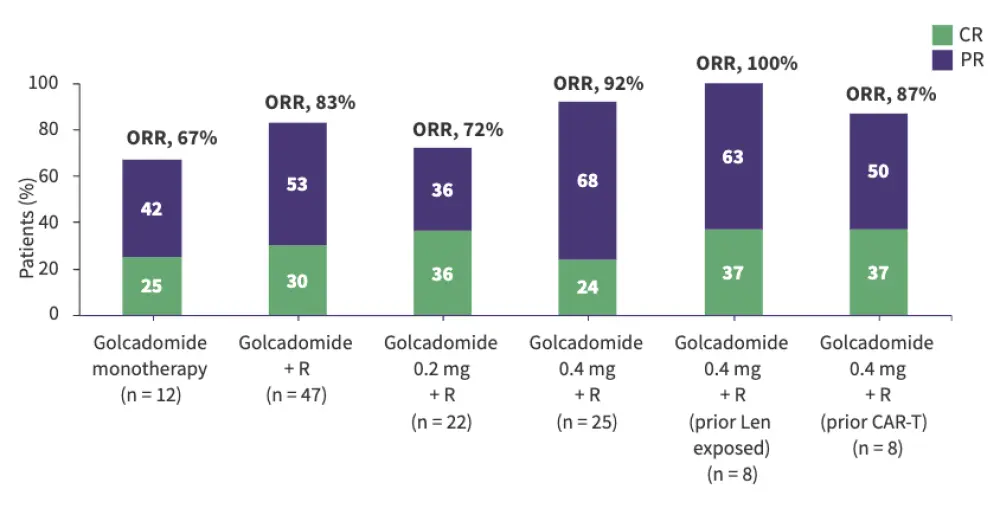
Figure 3. Efficacy of golcadomide in R/R NHL: Results from the CC-99282-NHL-001 trial
A. DLBCL cohort†
B. DLBCL cohort†
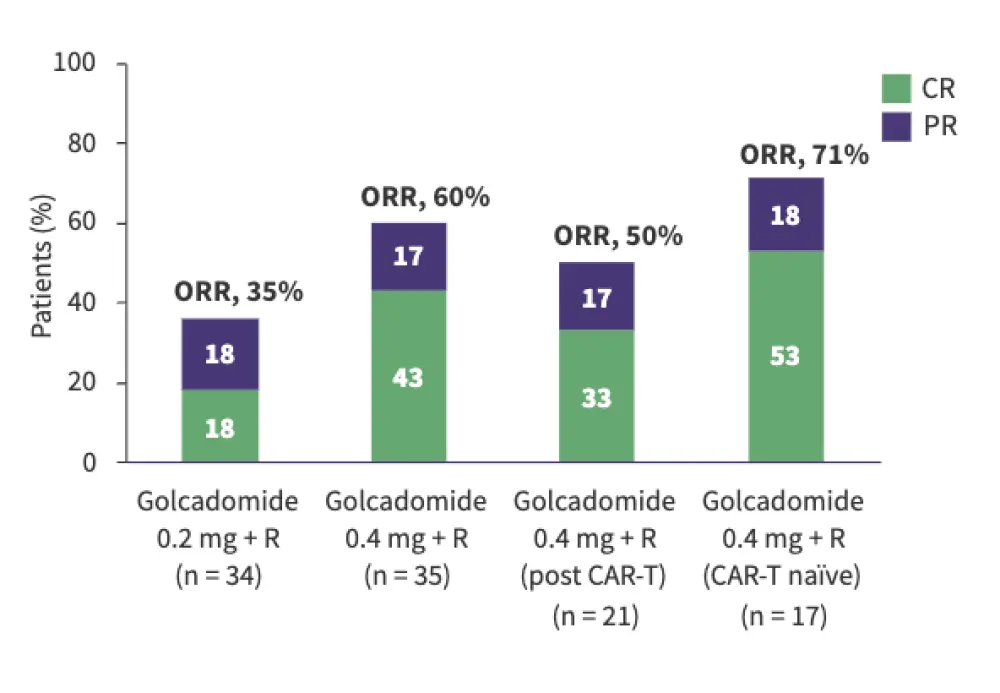
Salles also presented an overview of the ongoing phase III GOLSEEK-1 (NCT06356129) and phase II GOLSEEK-2 (NCT06425302) trials of golcadomide. The presentation was followed by a panel discussion focused on the evolving role of CELMoDs in lymphoid malignancies, particularly their integration into current treatment paradigms across lymphoma subtypes.
Key learnings
Mechanism of action of CELMoDs
CELMoDs bind cereblon, a substrate receptor in E3 ubiquitin ligase complexes, inducing degradation of transcription factors like Ikaros and Aiolos that are vital for B-cell survival and immune modulation.
Compared with immunomodulatory drugs, CELMoDs have higher cereblon affinity and broader immunostimulatory effects, improving T/NK-cell activity and tumor cytotoxicity.
Clinical activity and safety of CELMoDs
Iberdomide was evaluated in the phase I/II CC-220-NHL-001 trial in heavily pretreated relapsed/refractory (R/R) lymphomas.
Key outcomes:
The ORR with iberdomide monotherapy was 38%; this increased when iberdomide was combined with rituximab or obinutuzumab.
Adverse events included neutropenia, anemia, thrombocytopenia, constipation, diarrhea, asthenia, pyrexia, COVID-19, cough, and back pain.
Golcadomide was evaluated in the phase I/II CC-99282-NHL-001 trial in patients with R/R FL and R/R DLBCL. Golcadomide showed promising activity as a monotherapy and in combination with rituximab, including in chimeric antigen receptor (CAR) T-cell therapy- and lenalidomide-exposed patients.
Key outcomes:
Golcadomide monotherapy:
In patients with R/R FL, ORR was 67%.
In patients with R/R DLBCL, ORR was 35%.
Golcadomide in combination with rituximab:
In patients with R/R FL, ORR was 72–100% across the subgroups assessed.
In patients with R/R DLBCL, ORR was 50–71% across the subgroups assessed.
Adverse events included dose-dependent neutropenia, febrile neutropenia, anemia, thrombocytopenia, constipation, fatigue, asthenia, and pyrexia.
Ongoing trials of golcadomide
The phase III GOLSEEK-1 trial is evaluating golcadomide + rituximab-cyclophosphamide-doxorubicin-vincristine-prednisone (R-CHOP) vs placebo + R-CHOP in patients with newly diagnosed high-risk large B-cell lymphoma.
This study is recruiting at 59 sites in 13 countries, across North America, Europe, South America, Australia, and Asia.6
The phase II GOLSEEK-2 trial is evaluating golcadomide + rituximab (R) vs R-CHOP or obinutuzumab-based regimens in patients with untreated FL.
This study is recruiting at 319 sites in 39 countries, across the United States, Europe, Latin America, and East Asia.5
Additional phase I/II trials are exploring golcadomide combinations with chemotherapy, bispecific antibodies, and CAR T-cell therapies.
Panel discussion highlights
Positioning: CELMoDs show synergy with anti-CD20 antibodies such as rituximab and obinutuzumab. Their ability to improve antibody-dependent cellular cytotoxicity (ADCC) positions them as strong partners in combination regimens. In aggressive lymphoma like DLBCL, CELMoDs may serve as immunomodulatory enhancers when combined with standard chemotherapy (e.g. R-CHOP or Polatuzumab-R-CHP). In indolent lymphomas such as FL, CELMoDs hold potential as components of chemo-free regimens, replacing lenalidomide in combinations such as Rituximab-CELMoDs.
Subtype activity: CELMoDs appear to be active across both GCB and ABC subtypes of DLBCL, unlike lenalidomide which tends to show greater efficacy in ABC/non-GCB subtypes. This broader activity may be due to CELMoDs’ primary mechanism of improving immune effector cell function rather than direct tumor cytotoxicity alone.
Safety profile: Neutropenia was manageable; thromboembolism and teratogenicity risks remain low but require monitoring. An optimized CELMoD dosing schedule is essential to ensure it does not interfere with the timely and full delivery of chemotherapy, especially in first-line settings where maintaining the planned R-CHOP regimen without delays is critical.
Comparative efficacy: While head-to-head comparisons are lacking, early data suggest that golcadomide may have promising single-agent activity vs lenalidomide, particularly in R/R FL. CELMoDs have demonstrated responses even in patients previously treated with lenalidomide or CAR T-cell therapy, suggesting non-cross-resistance and a distinct immunological mechanism of action.
Potential in mantle cell lymphoma: Golcadomide could be promising in mantle cell lymphoma, especially where rituximab + obinutuzumab (R2) has limited efficacy.
Future strategies: With a competitive treatment landscape (e.g. bispecific antibodies, tafasitamab), CELMoDs must show advantages in efficacy or tolerability to secure first-line or post-CAR T-cell therapy treatment option.
Conclusion
CELMoDs, particularly golcadomide, show promise in both FL and DLBCL, with encouraging efficacy in heavily pretreated populations. Mantle cell lymphoma was noted as another potential area of application, especially given the limited success of lenalidomide in this subtype. Ongoing randomized trials will define their future role, especially in comparison or combination with standard treatments such as lenalidomide, anti-CD20 antibodies, and chemotherapy.
This educational resource is independently supported by Bristol Myers Squibb. All content was developed by SES in collaboration with an expert steering committee; funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Ulrich Jäger
Ulrich Jäger Grzegorz Nowakowski
Grzegorz Nowakowski Michael Dickinson
Michael Dickinson Marek Trněný
Marek Trněný Catherine Thieblemont
Catherine Thieblemont Gilles Salles
Gilles Salles Francesc Bosch
Francesc Bosch Sonali Smith
Sonali Smith Miles Prince
Miles Prince
