All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
American Society for Transplantation and Cellular Therapy recommendations for allo-HSCT in MDS
The American Society for Transplantation and Cellular Therapy (ASTCT), previously known as the American Society for Blood and Marrow Transplantation, published a review in 2009 on allogeneic hematopoietic cell transplantation (allo-HSCT) in patients with myelodysplastic syndromes (MDS). Since then, the diagnostic, prognostic, and treatment landscape in MDS has advanced significantly, with novel therapeutic agents and improved allo-HSCT practices.
The MDS Hub has previously reported on guidelines for allo-HSCT in the treatment of pediatric acute myeloid leukemia and MDS. An updated review on allo-HSCT in patients with MDS, including treatment recommendations from the ASTCT was recently published by DeFilipp et al.1 in Transplantation and Cellular Therapy, which we are pleased to summarize here.
Methods
An expert panel of 20 physicians and members of the ASTCT Committee on Practice Guidelines reviewed the evidence. A total of 140 studies were included and organized into subtopics based on frequently asked questions considered important. Grading was completed for all articles by two experts, with final grading and recommendations made only after full consensus was achieved amongst the panel members.
Recommendations
The consensus recommendations relating to frequently asked questions on transplantation indications, patient- and disease-specific factors, treatment-specific considerations, conditioning regimens, alternative donors, and posttransplantation considerations in patients with MDS are summarized in Figure 1, with further details in the following text.
Figure 1. ASTCT treatment recommendations for patients with MDS*
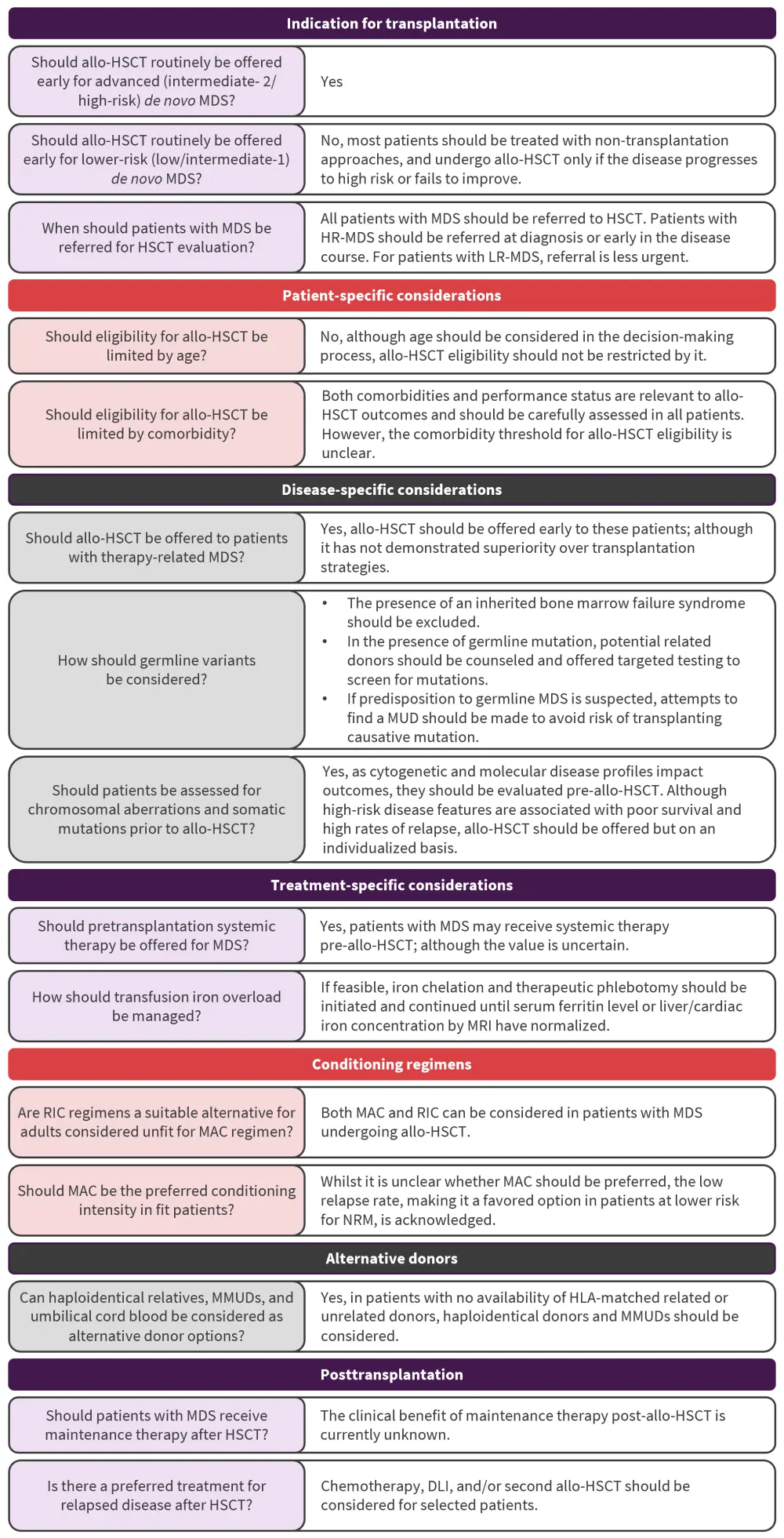
allo-HSCT, allogeneic hematopoietic stem cell transplantation; ASTCT, American Society for Transplantation and Cellular Therapy; DLI, donor lymphocyte infusion; HLA, human leukocyte antigen; HR, higher-risk; LR, lower-risk; MAC, myeloablative conditioning; MDS, myelodysplastic syndromes; MMUD, mismatched unrelated donor; MRI, magnetic resonance imaging; MUD, matched unrelated donor; NRM, non-relapse mortality; RIC, reduced intensity conditioning.
*Adapted from DeFilipp, et al.1
Summary of key evidence
Indications for transplantation
What is the role of allo-HSCT in MDS?
In patients with intermediate or high-risk MDS, allo-HSCT is a recommended approach. However, there is uncertainty around the optimal timing for transplant, with decisions currently based on the International Prognostic Scoring System (IPSS) alongside Markov decision models. Retrospective decision analyses and large prospective donor versus no-donor trials have shown superior treatment outcomes in patients with higher-risk MDS (HR-MDS) undergoing allo-HSCT compared with non-transplantation approaches, whereas delaying allo-HSCT in lower-risk IPSS groups until disease progression maximized overall survival (OS). On the contrary, patients in higher-risk groups have shown benefit from allo-HSCT at the time of diagnosis. Improved life expectancy and quality-adjusted life expectancy in patients with HR-MDS has been reported with reduced-intensity conditioning (RIC) in patients aged ≥60 years. Furthermore, allo-HSCT recipients have been found to have improved event-free survival at 3 years versus those receiving continuous azacitidine (34% vs 0%; p < 0.0001).
What is the role of allo-HSCT in lower-risk MDS?
In general, patients with lower risk-MDS should not be routinely offered allo-HSCT. These patients have better outcomes following transplant, as they are at lower risk of disease relapse; although they often have a disease- or patient-related variable that is of high clinical concern for the treating physician. Although decision analyses show an association between longer life expectancy and non-transplantation approaches, there are some limited data supporting higher complete response rates and improved OS in patients receiving allo-HSCT versus those receiving non-transplantation therapies.
When should patients with MDS be referred for allo-HSCT evaluation?
No studies have addressed the ideal timing for referral for allo-HSCT evaluation, with guidance coming from data from decision analyses and prospective longitudinal evaluations. Patients with HR-MDS should be referred for human leukocyte antigen typing and initial discussion of allo‑HSCT at diagnosis or early in their disease course. Patients at lower risk can also be referred for an early discussion to pre-plan human leukocyte antigen typing and identify donor options. All patients with MDS should be referred for allo-HSCT and undergo an individualized risk assessment; although future studies to further refine specific allo-HSCT recommendations are needed.
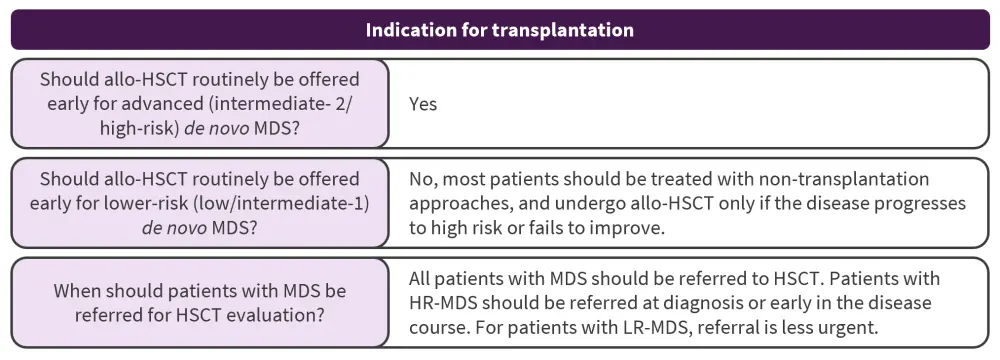
allo-HSCT, allogeneic hematopoietic stem cell transplantation; LR, lower-risk; MDS, myelodysplastic syndromes.
Patient-specific considerations
How does age impact the decision regarding transplantation?
The use of allo-HSCT was previously limited to younger patients, owing to the severity of treatment-related adverse events. However, the introduction of RIC and enhanced supportive care have enabled better transplant outcomes in older people. Although some evidence has shown that increasing age is correlated with worse non-relapse mortality (NRM) after allo-HSCT, other studies found no association with OS or NRM. Therefore, age alone cannot be considered as a measure for allo-HSCT eligibility. In addition, in patients aged ≥80 years, no recommendations can be made due to limited data.
How do comorbidities impact the decision regarding transplantation?
The impact of comorbidities and performance status on the outcomes of allo-HSCT should be carefully assessed in all patients. However, not all comorbidities carry the same risk making it unclear what the comorbidity threshold should be for eligibility for allo-HSCT. A formal geriatric assessment may be taken into consideration for transplantation decision after an individual assessment of benefits and risks has been completed.
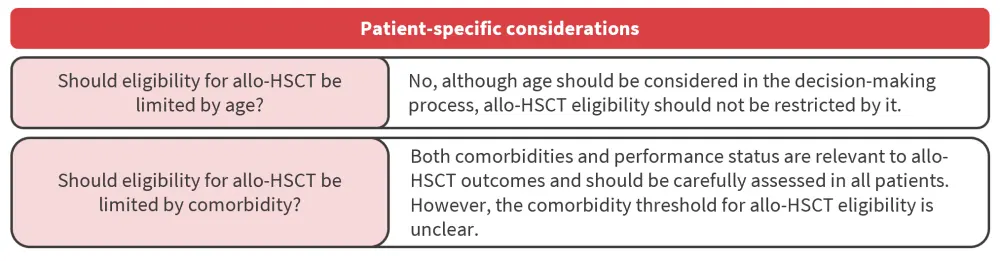
allo-HSCT, allogeneic hematopoietic stem cell transplantation.
Disease-specific considerations
What are specific considerations for therapy-related MDS?
Poor outcomes are associated with therapy-related MDS (t-MDS) compared with de novo MDS. However, few studies have investigated the role of allo-HSCT in this patient subgroup. Despite higher-risk features being associated with inferior transplantation outcomes, the effect of MDS mutation risk in these patients has not been adequately evaluated. Allo-HSCT should therefore be offered early to patients with t-MDS while noting that allo-HSCT has shown no advantages over non-transplantation approaches in this subgroup. Further studies evaluating how revised risk stratification approaches in t-MDS may impact the role of allo-HSCT in these patients are needed.
How should germline variants be considered?
Although data on the impact of germline mutations on allo-HSCT outcomes are limited, genetic testing is increasing in use. However, the presence of an MDS germline mutation is important to consider as it can impact the pre-HSCT assessment in younger patients.
How should chromosomal anomalies and somatic gene mutations be considered?
The decision to pursue allo-HSCT is influenced by the presence of high-risk disease chromosomal and molecular features and its impact on clinical outcomes. Limited evidence has shown cytogenetic risk to be associated with higher rates of relapse and mortality in patients post-allo-HSC, with chromosome 7 aberrations and monosomal karyotype associated with poor posttransplant OS and progression-free survival, with a stronger predictive value than complex karyotypes. Mutated TP53 was strongly associated with posttransplant OS (HR, 1.71; p < 0.001) and shorter time to relapse (HR, 2.03; p < 0.001) in a large analysis of 1,514 patients.
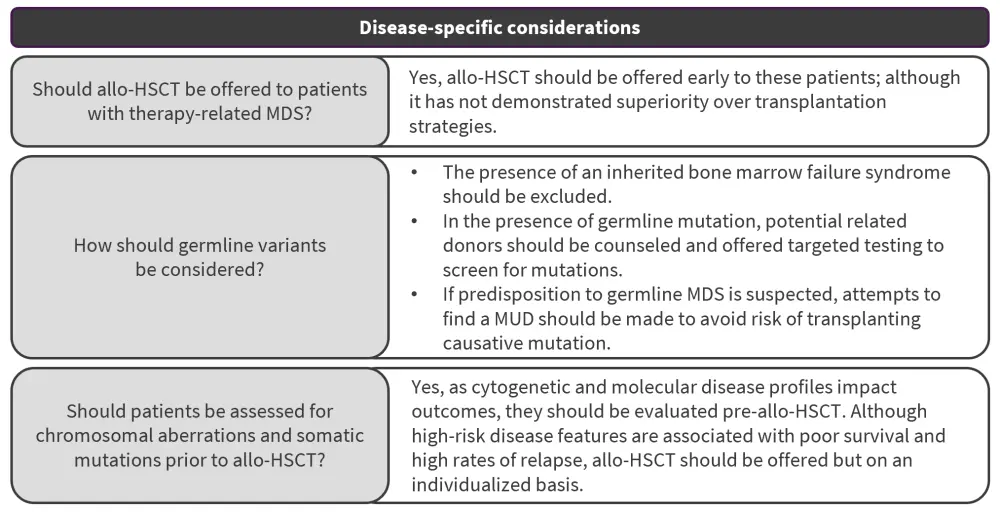
allo-HSCT, allogeneic hematopoietic stem cell transplantation; MDS, myelodysplastic syndromes.
Treatment-related considerations
What is the role of pretransplantation systemic therapy for MDS?
With most evidence taken from retrospective cohort studies with limited control populations, this is currently unknown. Reduced relapse rates and longer posttransplant disease-free survival is associated with low disease burden (<5% bone marrow blasts) at the time of allo-HSCT. However, it is not clear if the disease burden is a variable risk factor or due to treatment sensitivity of the inherent disease. In patients with hypomethylating agent failure, allo-HSCT has the potential to achieve long-term OS for selected patients (>5% bone marrow blasts), including those with progressive disease.
How should transfusion iron overload be managed?
Iron overload is a frequent occurrence and may increase the risk of organ failure and infection, evident by a meta-analysis that showed high serum ferritin level was associated with poor OS, NRM, and progression-free survival post-allo-HSCT. A prospective study in patients with MDS also showed a negative impact of serum ferritin levels on OS and relapse. However, data are lacking to guide any treatment recommendation, and iron chelation and therapeutic phlebotomy can be challenging to administer.
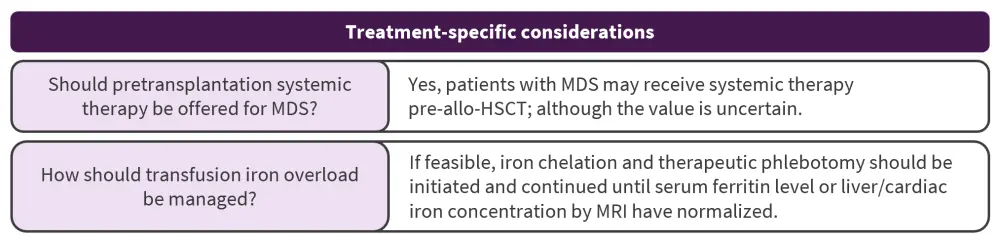
allo-HSCT, allogeneic hematopoietic stem cell transplantation; MDS, myelodysplastic syndromes; MRI, magnetic resonance imaging.
Conditioning regimen
How is conditioning intensity factored into pre-HSCT decision-making?
Randomized trials in younger patients fit for high-intensity therapy comparing allo-HSCT outcomes to conditioning intensity have found similar 1-year NRM and 2-year relapse-free survival (p = 0.58) or OS (p = 0.08) between RIC and myeloablative conditioning. Another trial showed that although treatment-related mortality was lower with RIC, relapse-free survival was significantly improved with myeloablative conditioning (p < 0.01). Additional trials have found an association between RIC and inferior disease risk index and outcomes in patients with measurable residual disease. However, disease-free survival was similar for high or very high disease index risk, irrespective of conditioning intensity.
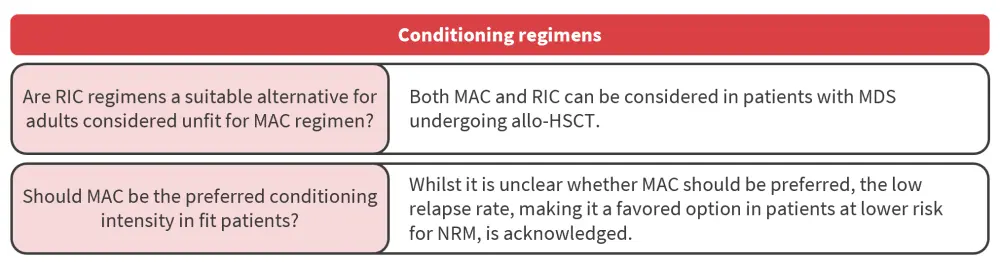
allo-HSCT, allogeneic hematopoietic stem cell transplantation; MAC, myeloablative conditioning; MDS, myelodysplastic syndromes; NRM, non-relapse mortality; RIC, reduced intensity conditioning.
Alternative donors
How are alternative donors considered in MDS pre-HSCT decision-making?
The practice of posttransplantation cyclophosphamide (PTCy) in recent years has increased the use of haploidentical donors. Studies comparing alternative donors in patients with MDS have shown haploidentical donor allo-HSCT along with the use of PTCy to be more beneficial, and PTCy was associated with better OS and lower 3-year NRM in patients with MDS undergoing mismatched related donor allo-HSCT.

HLA, human leukocyte antigen; MMUD, mismatched unrelated donor.
Posttransplant consideration
What are the indications for post-HSCT maintenance in MDS?
Efficacy assessment studies looking at the benefits of initiating therapy whilst patients remain in complete remission post allo-HSCT are limited. Early studies have established the safety of azacitidine and decitabine in the early posttransplantation setting in patients with acute myeloid leukemia. A phase II study highlighted that disease progression, patient preference, and toxicities may be some of the challenges in implementing prolonged hypomethylating agent maintenance after transplant.
How should relapse post-HSCT be managed?
High-risk disease features were associated with worse outcomes, and no clinical trials have identified an optimal treatment strategy. Further research on how disease- and transplantation-related factors might identify a sub-population who will benefit from post-HSCT therapy is needed. Patients with complex cytogenetic abnormalities or TP53 mutations are of particular interest.
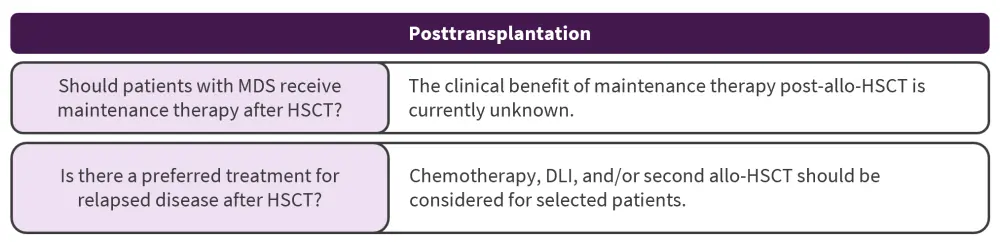
allo-HSCT, allogeneic hematopoietic stem cell transplantation; DLI, donor lymphocyte infusion; MDS, myelodysplastic syndromes.
Conclusion
The recommendations from the panel highlight the various advances in transplantation care enabling fit older patients with MDS to safely undergo allo-HSCT. The authors proposed that disease risk stratification approaches incorporating genetic mutational profiles will provide additional information on subpopulations with MDS who are likely to benefit the most. They also noted that disease relapse remains a major concern in the management of MDS; therefore, future trials should focus on including novel therapies both before and after allo-HSCT to improve clinical outcomes in patients with MDS.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


