All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
The role of the Nlrp3 inflammasome in hematological pathologies
The Nlrp3 inflammasome has recently gained a lot of attention due to its reported involvement in various hematological pathologies, including myeloproliferative neoplasms (MPN), leukemia, graft-versus-host disease (GvHD), and the myelodysplastic syndrome (MDS). Mariusz Ratajczak and colleagues published in Leukemia1, a comprehensive review on the Nlrp3 inflammasome and its relation to hematological diseases. This article is summarized below.
What is the Nlrp3 inflammasome?
Current evidence points to a role of the Nlrp3 inflammasome as a sensor of changes in the microenvironment and hematopoietic cell metabolic activity. Under homeostatic conditions, the Nlrp3 protein is located in an inactive state in the cell cytosol. Once activated, it forms clusters with adaptor proteins like the apoptosis-associated speck-like protein and procaspase-1 to create the Nlrp3 inflammasome. Due to the clustering proximity, procaspase-1 gets activated and in turn triggers cell death and the production of various proinflammatory cytokines, like interleukin (IL)-1β and IL-18. The Nlrp3 inflammasome exists in many immune cells, including macrophages, monocytes, granulocytes, dendritic cells, and lymphocytes. Recently, it was reported that the Nlrp3 inflammasome is also expressed in human hematopoietic stem and progenitor cells (HSPCs), triggering great scientific interest for its potential association with hematological malignancies.
How is the Nlrp3 inflammasome activated?
For the Nlrp3 inflammasome to get activated, both a priming signal (Signal 1) and an activating signal (Signal 2) are needed. Signal 1 is constantly present and is usually mediated by a lipopolysaccharide that is released from commensal microorganisms of the gastrointestinal tract. This causes the transcription of various inflammasome components, also referred to as ‘inflammasome priming’. Functional activation of the inflammasome occurs upon delivery of Signal 2. This activating signal is classified into either exogenous (pathogen-associated molecular patterns) or endogenous (damage-associated molecular patterns [DAMPs]) molecules induced by infected and/or damaged tissue (Figure 1).
Figure 1. Priming (Signal 1) and activation (Signal 2) steps needed for Nlrp3 inflammasome activation.1
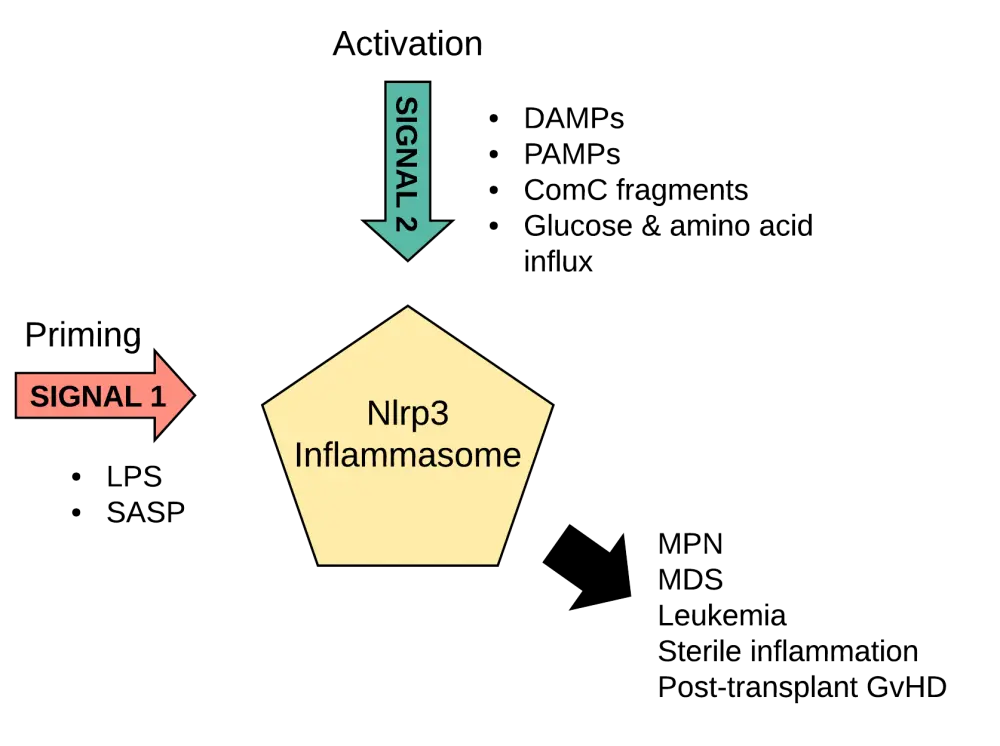
ComC, complement cascade; DAMPs, damage-associated molecular patterns; GvHD, graft-versus-host disease; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasms; PAMPs, pathogen-associated molecular patterns; SASP, senescence-associated secretory phenotype
Endogenous activation through DAMPs occurs in stress situations and when a tissue or organ is damaged with DAMPs, producing a so-called sterile inflammation. This sterile inflammation is also induced in the bone marrow after mobilization of HSPCs with the granulocyte colony-stimulating factor or a C-X-C motif chemokine receptor 4 antagonist, and after total body irradiation or administration of high doses of cytostatics for myeloablative conditioning.
What is the role of the Nlrp3 inflammasome on hematopoiesis?
Accumulating evidence indicates that the Nlrp3 inflammasome plays a key role in normal and malignant hematopoiesis. We hereby summarize the current evidence on the role of the Nlrp3 inflammasome in HSPC: (i) development and expansion, (ii) mobilization, migration, and engraftment (iii), as well as in aging-induced inflammation (inflammaging), (iv) metabolism-induced inflammation (metaflammation), (v) MDS, (vi) MPN, (vii) leukemia, and finally in (iv) posttransplantation GvHD.
i. Contribution of the Nlrp3 inflammasome to the early development and expansion of HSPCs?
Preliminary evidence indicates that the Nlrp3 inflammasome increases the hematopoietic potential of HSPCs. In support of this hypothesis, it has been shown that glucose influx in vertebrate embryos increases the proliferation of embryonic HSPCs which is dependent on Nlrp3 inflammasome activation and IL-1β release. Moreover, exposure of human induced pluripotent stem cells to Nlrp3 components increased their proliferation in vitro. Nevertheless, there is still a lack of in vivo data to support these observations.
ii. Nlrp3 inflammasome and HSPC mobilization, migration, and engraftment
Previous studies from the authors have shown that the Nlrp3 inflammasome is crucial for the mobilization of HSPCs, and other bone marrow stem cells, into the blood. This occurs via the release of purinergic signalling (eATP), activating the inflammasome and caspase-1 to release active IL-1β and IL-18. This cascade generates a state of sterile inflammation in the bone marrow and thus enables HSPC mobilization. Defects in any of these involved pathways significantly reduce HSPC mobilization.
Preliminary data indicate that HSPCs from Nlrp3 knockout mice have abnormal migration patterns in response to chemoattractants that are upregulated in the bone marrow following myeloablative preparation for HSCT (eATP, stromal-derived factor-1). The authors proposed that eATP acts as a paracrine or autocrine enhancer of donor HSPC lipid rafts, which facilitate chemoattraction and migration. In support of this hypothesis, data from Nlrp3 knockout mice have shown that HSPCs from these animals cannot successfully engraft in syngeneic wild type mice, due to defective lipid raft-mediated migration.
iii. Inflammaging
With physiological aging, a state of chronic low-level sterile inflammation exists systemically and in the bone marrow. In the bone marrow, aging leads to a gradual increase in myelopoiesis over lymphopoiesis. This seems to be at least in part modulated by the Nlrp3 inflammasome, as increased release of IL-1β and IL1-R following Nlrp3 activation with aging, leads to elevated production of cytokines that stimulate myelopoiesis in the bone marrow. In contrast, erythropoiesis is decreased with aging, possibly modulated by the cytokines released following Nlrp3 inflammasome activation. In fact, IL-18 together with IL-1a are known to inhibit erythroid colony formation, and IL-1R signalling decreases erythropoietin secretion.
These age-related changes in hematopoiesis that seem to be induced by the chronic sterile inflammatory state are known as ‘inflammaging’. Inflammaging occurs due to chronic cell replacement and stress in the absence of infections, and is mediated by cytokines and DAMPs. These in turn activate the Nlrp3 inflammasome which probably affects the aging bone marrow microenvironment in a way that it increases the risk of malignant hematopoiesis. Although the involvement of the Nlrp3 inflammasome in inflammaging is evident, its precise role remains to be determined.
iv. Metaflammation
Inflammaging can be further aggravated by an unhealthy nutritional lifestyle, which by itself can induce a low-grade chronic inflammation state. The combination of inflammaging and this metabolically induced inflammation has been termed as ‘metaflammation’. Metaflammation has been associated with the activation of an early innate immune response through the assembly of the Nlrp3 inflammasome. It is likely that excessive caloric intake may lead to increased Nlrp3 inflammasome activation, since it is known that glucose and amino acid uptake can act as inflammasome activators (Figure 1). It was observed that Nlrp3 deficiency decreases systemic inflammation and improves cellular metabolism and insulin resistance, further supporting the role of the Nlrp3 inflammasome in cell metabolism. Moreover, high glucose levels have been linked to the Nlrp3 inflammasome activation and subsequent release of IL-1β and IL-18.
But how do these effects of the Nlrp3 inflammasome on cellular metabolism affect hematopoiesis? Following the revelation that the innate immunity complement factors C3 and C5 are also expressed and cleaved inside T cells (complosome), and the fact that the complement cascade activation has been linked to metabolic change sensing via the mammalian target of rapamycin C1 (mTORC1), a new concept of a complosome-metabolism-Nlrp3 inflammasome axis has emerged. This mTOR regulated T-cell complosome activation (via C3a and C5a) seems to provide the appropriate activating signals for the Nlrp3 inflammasome, as mTOR signalling inhibition by rapamycin completely blocks the inflammasome activation and IL-1β production in T cells. Interestingly, the authors provide preliminary data in this article showing that HSPCs also express C3 and C5 mRNA in vitro, supporting the existence of the complosome-metabolism-Nlrp3 inflammasome axis in these cells too. However, the effects of this metabolically induced inflammasome activation on HSPC biology and hematopoiesis are yet to be determined.
v. What is the current role of Nlrp3 in MPN?
MPN are hematological malignancies that are characterized by chronic inflammation and can transform to MDS or acute myeloid leukemia. In MPN, the bone marrow inflammation is mediated by the existence of the neoplastic clones, as allogeneic stem cell transplantation completely restores hematopoiesis and removes bone marrow inflammation. The potential role of the inflammasome in this clone-induced bone marrow inflammation is yet unclear, with a great need for more in-depth studies to address any likely associations.
vi. Nlrp3 inflammasome and MDS
MDS is hallmarked by a dysplastic bone marrow with defective hematopoiesis. The events leading to this clinical presentation are complex, but seem to involve the abnormal activation of innate immunity and the presence of a ‘’smouldering’’ proinflammatory state in the bone marrow that contribute to the expansion of the MDS clone and hematopoietic cell dysfunction. The potential link between the Nlrp3 inflammasome and MDS is evident through recent data suggesting that the activation of the inflammasome enhances bone marrow inflammation, hematopoietic cell damage, and death (DAMP-induced pyroptosis), as well as myeloid-derived suppressor cell proliferation.
In fact, in the blood samples of MDS patients, the following Nlrp3 inflammasome activators have been reported in significantly elevated amounts:
- S100A8 (DAMP)
- S100A9 (DAMP)
These DAMPs mainly form heterodimers and produce both Signals 1 and 2 (see Figure 1) for the functional activation of the inflammasome. Moreover, they have been shown to induce pyroptosis, an inflammasome-mediated cell death, in HSPCs from patients with MDS. Pyroptosis leads to cell swelling and rupture with a substantial release of DAMPs and pro-inflammatory cytokines like IL-1β and IL-18. These modulators recruit more immune cells further perpetuating the inflammatory state in the bone marrow milieu.
Preclinical studies in MDS mouse models have reported that S100A9 neutralization or inhibition of inflammasome-mediated pyroptosis restores effective hematopoiesis. These findings indicate that the Nlrp3 inflammasome acts as a driver of the MDS phenotype in vivo.
Further studies are needed to uncover the exact mechanisms involved in this MDS-mediated Nlrp3 inflammasome activation, and its contribution to disease pathogenesis and/or progression.
vii. Nlrp3 inflammasome and leukemia
Persistent chronic inflammation in the bone marrow due to MDS or MPN may lead to leukemic transformation. This is an important pathogenic pathway in elderly patients with leukemia, and it could be partially driven by the activation of the inflammasome that normally occurs with age. Moreover, preliminary data have indicated that the Nlrp3 inflammasome is crucial for the migration and spread of leukemic cells throughout the body. Nevertheless, the precise role of the Nlrp3 inflammasome in the pathogenesis of leukemia is still unclear.
viii. Nlrp3 and posttransplant GvHD
During posttransplant GvHD, the following DAMPs have been reported to activate the Nlrp3 inflammasome:
- Purinergic signalling in the form of eATP
- Crystalized uric acid released from ischemic tissues
It has been suggested that IL-1β release due to the inflammasome activation, drives allogeneic T-cell differentiation to T helper cells, which are widely implicated in GvHD induction. In support of this observation, elevated capsase-1 and IL-1β levels were found in white blood cells and intestinal lesions of patients with GvHD. Moreover, IL-18, which is also released from the activated Nlrp3 inflammasome, has also been shown to increase in patients with GvHD. These findings support the need for further in-depth studies targeting the Nlrp3 inflammasome as a potential treatment for GvHD.
Conclusion
Although the precise role of the Nlrp3 inflammasome in hematological malignancies is still unclear, there is a plethora of preclinical evidence highlighting its effect on hematopoiesis. These findings warrant the need for more detailed preclinical and clinical studies to further explore the potential of Nlrp3 as a therapeutic target for MPN, MDS, leukemia, and GvHD. In fact, the fist in-human clinical trials of multiple Nlrp3 inflammasome inhibitors, including MCC950, are soon to start.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

