All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Real-world validation of the IPSS-M risk stratification model for MDS and comparison to IPSS-R
Featured:
The Molecular International Prognostic Scoring System (IPSS-M) is a new risk stratification model for patients with myelodysplastic syndromes (MDS) that was recently proposed to improve conventional risk stratification from the Revised International Prognostic Scoring System (IPSS-R).1,2
The IPSS-M model, which has been previously covered by the MDS Hub, includes mutations in 31 genes, along with cytogenetics, bone marrow blasts, hemoglobin, and platelet counts.2,3 This new model reflects the importance of molecular analysis when performing risk stratification. Two other personalized prediction models by Nazha et al.4 and Bersanelli et al.5 also include recurrently mutated genes.3 The IPSS-M model may also improve risk stratification within clinical trials.6
Several presentations at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition discussed the implementation of the IPSS-M and compared the prognostic ability of this model with the IPSS-R, as well as the original International Prognostic Scoring System (IPSS) and alternative personalized prediction models. Below, we summarize presentations by Sauta1, Aguirre2, Baer3, and Santini6 assessing the predictive capabilities of IPSS-M in real-world populations.
Validation of IPSS-M in a European population1
Study design and patient characteristics
This retrospective analysis included 2,876 patients with MDS from 21 European centers affiliated with the GenoMed4All consortium. Clinical, cytogenetic, and molecular profile was determined for included patients before initiating disease-modifying treatment and/or at diagnosis. The median age of this cohort was 68 years (range, 18–96 years) and the median follow-up time was 37.5 months (range, 36.2–38.8 months). IPSS-M and IPSS-R models were evaluated for their predictive abilities using the concordance index (c-index).
Key findings
- In total, 82.4% of patients had ≥1 somatic mutation on the evaluated 31 IPSS-M genes.
- The IPSS-M model showed more accurate prediction versus IPSS-R in terms of survival probability (IPSS-M c-index, 0.81; IPSS-R c-index, 0.74) and leukemia-free survival (LFS) probability (IPSS-M c-index, 0.89; IPSS-R, 0.76).
- This remained true in patients without detectable IPSS-M mutations within this cohort for survival probability (IPSS-M c-index, 0.89; IPSS-R c-index, 0.73) and LFS probability (IPSS-M c-index, 0.97; IPSS-R c-index, 0.81).
- 46% of patients were restratified from IPSS-R, with 23.6% of patients upstaged and 22.4% downstaged.
- Among patients who were IPSS-R very low or low risk (n = 1,099), 19.5% were upstaged.
- Among patients who were IPSS-R intermediate risk (n = 610), 29% were downstaged and 26% were upstaged.
- 964 patients received allogenic hematopoietic stem cell transplantation and among these patients, IPSS-M better predicted post-transplant survival probability (IPSS-M c-index, 0.76; IPSS-R c-index, 0.60) and cumulative incidence of relapse (IPSS-M c-index, 0.89; IPSS-R c-index, 0.70).
- 268 patients who were not eligible for allogeneic hematopoietic stem cell transplantation were treated with hypomethylating agents (HMA), with an overall response of 42%, which was not significantly different among IPSS-M risk categories.
- Response duration and probability of survival were inversely related to the IPSS-M risk model in patients who received treatment with HMA.
- To determine the robustness of IPSS-M, a minimum set of 15 genes were defined to ensure risk prediction accuracy >70%: ASLX1, CBL, DNMT3A, ETV6, EZH2, FLT3, IDH2, MLLPTD, NPM1, NRAS, RUNX1, SF3B1, SRSF2, TP53multihit, and U2AF1.
Real-world evidence of IPSS-M in this comprehensive European study supports the implementation of IPSS-M to help improve MDS prognostication and potentially support better patient selection for HSCT.
Clinical implementation of IPSS-M in the US2
Study design and patient characteristics
This study included 2,355 patients with MDS treated at the Moffit Cancer Center and with available clinical and molecular data. Correlative analysis between IPSS-R and IPSS-M scores and outcome predictions were performed on LFS, overall survival (OS), and leukemic transformation. Model discrimination was evaluated using the c-index. The median age of this cohort was 70 years (range, 18–92 years) and the median duration of follow-up was 4.6 years (range, 4.4–4.9 years). The cohort was enriched with high-risk and very high-risk patients compared with the IPSS-M discovery cohort.
Key findings
- IPSS-M improved discrimination compared with IPSS-R for LFS (2.3-point increase in c‑index), OS (2.0-point increase in c‑index), and leukemic transformation (1.5-point increase in c‑index).
- A five-to-five mapping between IPSS-R and IPSS-M subgroups led to 45% of patients being restratified (Table 1).
- 10.5% of patients who were IPSS-R lower-risk and intermediate-risk were restratified into IPSS-M higher-risk strata.
- The restratification of patients from IPSS-R intermediate to higher-risk strata in IPSS-M was associated with notable differences in survival outcomes.
Table 1. Restratification from IPSS-R to IPSS-M*
|
Risk category restratification, % (n = 1,056) |
Very low |
Low |
Intermediate |
High |
Very high |
|
IPSS-M, Molecular International Prognostic Scoring System; IPSS-R, Revised International Prognostic Scoring System. |
|||||
|
Downstaged |
0 |
2.7 |
7.9 |
14.7 |
18.6 |
|
Upstaged |
73.6 |
44.3 |
39.4 |
41.5 |
0 |
Within this large study cohort, the implementation of IPSS-M showed improved discrimination capability versus IPSS-R in terms of LFS, OS, and leukemic transformation alongside providing relevant impact on risk restratification and survival outcomes in the clinical setting.
Comparison of IPSS-M to IPSS-R and two other novel personalized prediction tools3
Study design and patient characteristics
This study aimed to first validate IPSS-M when compared with IPSS-R in a cohort of 626 patients with MDS characterized by whole-genome sequencing. Following this first analysis, IPSS-M was compared with the Nazha et al.4 and Bersanelli et al.5 risk stratification models in a cohort of 432 patients. Patients were diagnosed with MDS between 2005 and 2020 and the median age was 73 years (range, 23–93 years), while the median follow-up was 9.5 years.
Key findings
- Significant separation was observed between IPSS-M risk groups in terms of OS (p < 0.001), LFS (p < 0.001), and leukemic transformation (p < 0.001).
- 19% of patients were downstaged and 25% of patients were upstaged when comparing IPSS‑M with IPSS‑R.
- When comparing IPSS-M from the Bersanelli score and the Nazha model, six genes were found to overlap between all models: ASXL1, RUNX1, SF3B1, SRSF2, STAG2, and TP53.
- In total, the Bersanelli score required 54 parameters, the Nazha model required 19 parameters, and the IPSS-M required 37 parameters.
- The IPSS-M model does not consider age, whereas the Bersanelli score gives the second strongest weight to age, and age is of intermediate importance for OS in the Nazha model.
- Based on the c-index, the Nazha model best predicted OS, followed by the Bersanelli score, IPSS-M, and finally the IPSS-R score.
- In terms of leukemic transformation, the Nazha model had the highest c-index, followed by IPSS-M, IPSS-R, and finally the Bersanelli score.
This comprehensive comparative study highlighted the added value of molecular parameters included in the IPSS-M versus the IPSS-R in terms of improved predictive power and showed key strengths and differences between the three most recent predictive scores.
IPSS-M in patients receiving intravenous sabatolimab added to HMA6
Study design and patient characteristics
This analysis compared IPSS, IPSS-R, and IPSS-M scoring systems within the overall patient population from the phase II STIMULUS-MDS1 (NCT03946670) and phase III STIMULUS-MDS2 (NCT04266301) clinical trials, which have been previously covered by the MDS Hub. These trials investigated sabatolimab and HMA in patients with higher-risk MDS (Figure 1).
Figure 1. STIMULUS-MDS1 and STIMULUS-MDS2 study designs*
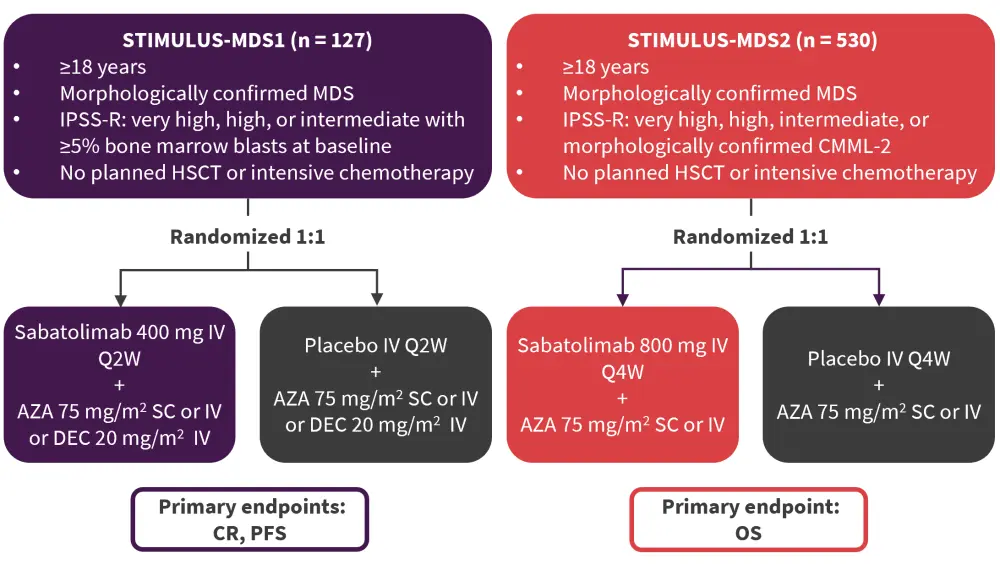
AZA, azacitidine; CMML-2, chronic myelomonocytic leukemia-2; CR, complete remission; DEC, decitabine; HSCT, hematopoietic stem cell transplant; IPSS-R, Revised International Prognostic Scoring System; IV, intravenous; MDS, myelodysplastic syndromes; OS, overall survival; PFS, progression-free survival; Q2W, every two weeks; Q4W, every four weeks; SC, subcutaneous.
*Adapted from Santini.6
Disease characteristics from the two studies were reported, with patients with chronic myelomonocytic leukemia-2 excluded from analyses. Patient populations from both trials were pooled and assessed for the migration of patients between the IPSS, IPSS-R, and IPSS-M scoring systems. The median age in the STIMULUS-MDS1 trial was 73 years and 71 years in the STIMULUS-MDS2 trial.
Key findings
- Bone marrow blasts of 10–20% were associated with higher-risk categories, with the majority very high-risk in the IPSS-R and IPSS-M models, but a sizeable portion of patients with low blast counts were also classified as very high-risk.
- When comparing IPSS-R with IPSS-M, of the patients with intermediate-risk IPSS-R, 22.2% were upstaged to high-risk and 21.5% were upstaged to very high-risk in IPSS-M.
- 52.2% of patients with high-risk IPSS-R were upstaged to very high-risk IPSS-M.
- 7.6% of patients with very high-risk IPSS-R were downstaged to high-risk IPSS-M.
Preliminary results obtained by this large, randomized-controlled study population underline the need for higher IPSS-M risk categories identification by molecular and cytogenetic assessment at baseline to help guide improved treatment decisions in MDS.
Conclusion
These studies show the relevant impact of molecular assessment on risk stratification, with the incorporation of molecular data in the IPSS-M model leading to improved discrimination of survival outcomes over IPSS-R. These recent findings may in turn lead to higher accuracy in prognostic assessments and ultimately benefit therapeutic decision-making for patients with MDS.
Expert opinion from Theo de Witte
The diagnosis of MDS is based on morphological, clinical, and cytogenetic features. The 5q− syndrome is the only genetic diagnostic category in the most recent WHO classification.
Is there confusion between WHO and IPSS-M classifications of MDS?
The prognosis of patients with MDS varies from a few months to more than 10 years. The revised International Prognostic Scoring System (IPSS-R) is based on the severity of the blood cytopenias, percentage of marrow blasts, and the cytogenetic risk categories. This most frequently used scoring system is usually adjusted for age, an important addition in view of the average high age of 75 years of these patients. The IPSS-R has been developed in a cohort of untreated patients, which may overestimate or underestimate the prognosis after treatment with interventions of specific activity such as lenalidomide in patients with 5q deletion.
Next-generation sequencing, usually performed with a selected set of 30–40 genes, has been reported to contribute to the clinical outcome of patients with MDS. Around 7 genes appeared to have a negative impact on outcome after adjustment for age and IPSS-R: ASXL1, EZH2, IDH2, NRAS, RAD21, RUNX1, STAG2, and biallelic TP53.
SF-3B1 is the only mutation with a positive prognostic impact on outcome. The International Working Group (IWG) proposed as diagnostic criteria for MDS with mutated SF3B1: cytopenias according to standard criteria, isolated erythroid or multilineage dysplasia with or without ring sideroblasts, and <5% marrow blasts and <2% blood blasts. This subgroup has a very prolonged survival >80 months and a low-risk of developing leukemia. MDS with isolated SF3B1 mutations are associated with a better prognosis than SF3B1-mutated MDS with 5q− or other mutations, including RUNX-1, biallelic TP53 mutations, and complex karyotype. The number of mutations and the variant allele frequency has a prognostic impact on outcome with the exception of isolated SF3B1 mutations. It is important to realize that patients with SF3B1-mutated MDS with clonal cytopenia of undetermined significance, so-called CCUS, develop almost invariably clinically overt RS-MDS.
86% of patients in the IWG IPSS-M studies have been classified across 17 non-overlapping molecular subgroups with a large variation in size of 1–15%; 6% of patients have no recognizable oncogenic lesions. These are usually young female patients with single-lineage MDS.
Adverse mutations are associated with short overall survival and high-risk of progression to overt leukemia, even in patients who are classified in the lower and intermediate-risk IPSS-R categories. Biallelic TP53 mutations are often associated with DNMT3A and U2AF1 mutations.
The intermediate-risk categories contain several specific subgroups. The subgroup with monocyte counts ranging from 0.5–0.9 × 109/L, but >10% peripheral monocytes and trilineage dysplasia, is characterized by TET2 mutations with or without SRSF2 mutations and WT SF3B1. RARS-T is associated with SF3B1 and JAK2 mutations; LR-MDS with trilineage dysplasia and cytopenias is associated with TET-2 mutations and WT SRSF2.
The IWG IPSS-M classification is in its final stage of development. The most recent update has been presented at ASH. It is important to realize that this group is based on >3,000 untreated patients, while the great majority of patients will be treated based on specific characteristics, including molecular data. The currently available drugs including ESA, lenalidomide, HMA, and luspatercept will be extended by TP53-specific drugs (APR-246) in the near future, and other specific drugs under investigation with or without a fast-track designation by FDA.
It is clear that MDS is involved in a rapidly changing world with promising prospects for our patients.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Theo de Witte
Theo de Witte
