All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Post-hoc analysis of combination therapies treosulfan and fludarabine, and busulfan and fludarabine, in patients with MDS
Currently, allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only curative option for patients with myelodysplastic syndromes (MDS). Previous phase III trials, including the NCT00822393 trial, have evaluated reduced intensity conditioning (RIC) involving treosulfan or busulfan plus fludarabine in elderly patients with acute myeloid leukemia (AML) and MDS, and have been reported on the AML Hub here. Due to a more acceptable toxicity profile associated with the RIC regimen, an increased number of patients in the 50–70 age range can be considered for allo-HSCT. Eleni Gavriilaki, from the George Papanikolaou General Hospital of Thessaloniki, Thessaloniki, GR, further discusses here, how these new conditioning combinations are impacting transplant outcomes.
During the 48th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT), Matthias Stelljes, University of Münster, Münster, DE, presented a post-hoc subgroup analysis of a phase III study (NCT00822393), which compared the combination treosulfan and fludarabine (FT) with busulfan and fludarabine (FB), in elderly and/or comorbid patients with MDS to understand the impact within this subgroup.1 We summarize the results below.
Study design
Patients aged between 50 and 70 years, or aged ≥18 years with a hematopoietic cell transplantation comorbidity index (HCT-CI) >2, with a Karnofsky performance index of ≥60%, and having received either a matched-related or -unrelated donor graft were included in the trial. An overview of the study design is shown in Figure 1.
Figure 1. Study design overview*
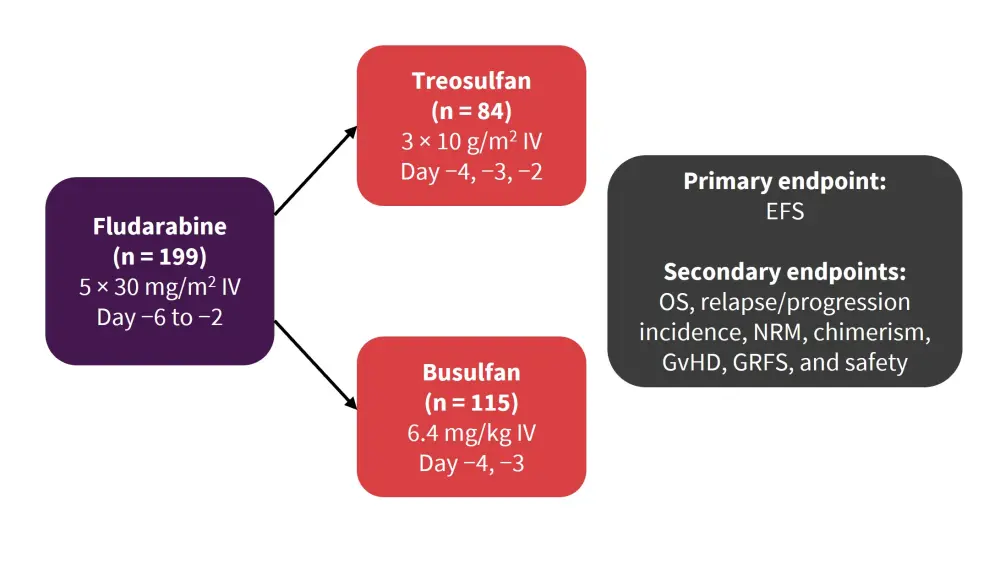
EFS, event-free survival; GRFS, GVHD-free/relapse-free survival; GvHD, graft-versus-host disease; NRM, nonrelapse mortality; OS, overall survival.
*Adapted from Stelljes et al.1
Results
The patient and disease characteristics were balanced across the treatment groups. For example, the median age of patients for busulfan and treosulfan was 61 and 60, respectively, and the median bone marrow (BM) blast count was 5% for both arms (Table 1).
Table 1. Disease and patient characteristics*
|
BM, bone marrow; IPSS-R, International Prognostic Scoring System-Revised; PB, peripheral blood. |
|||
|
Characteristic |
Busulfan |
Treosulfan |
Total |
|---|---|---|---|
|
Male/female, % |
70.4/29.6 |
69.0/31.0 |
69.8/30.2 |
|
Median age (range) |
61 (41–70) |
60 (42–70) |
61 (41–70) |
|
<50 years, % |
2.6 |
7.1 |
4.5 |
|
>49 years, % |
97.4 |
92.9 |
95.5 |
|
Median BM blast count, % (range) |
5 (0–19) |
5 (0–19) |
5 (0–19) |
|
IPSS-R risk, % |
|||
|
Very low/low |
17.4 |
23.9 |
20.1 |
|
Intermediate |
30.4 |
21.4 |
26.6 |
|
High |
24.3 |
26.2 |
25.1 |
|
Very high |
27.8 |
28.6 |
28.1 |
|
Donor type, % |
|||
|
Matched-related donors |
15.7 |
20.2 |
— |
|
Matched-unrelated donors |
84.3 |
79.8 |
— |
|
Stem cell source, % |
|||
|
PB |
98.3 |
98.8 |
— |
|
BM |
1.7 |
1.2 |
— |
Neutrophil and platelet recovery was similar in both arms included in this analysis, but more patients in the treosulfan/fludarabine arm had donor cell chimerism compared with the busulfan/fludarabine arm at Day 28 (90.2 vs 77.2%), and Day 100 (84.4 vs 76.0%). Kaplan-Meier estimates of event-free survival (EFS) at 2 years was significantly better for treosulfan/fludarabine compared to busulfan/fludarabine (68.1 vs 48.2%, respectively; (p = 0.0479792; Table 2). Other endpoints also demonstrated more favorable outcomes for the treosulfan/fludarabine treatment group (Table 2), though these were not significant. High and very high-risk patients had a significantly improved EFS for treosulfan/fludarabine compared to busulfan/fludarabine at 67.2% and 30.5%, respectively. In subgroup analysis, patients with higher blast counts at baseline were found to have a significantly better graft-versus-host disease (GvHD)-free/relapse-free survival (GRFS) outcome with treosulfan treatment than those patients treated with busulfan (baseline blast count ≥10–19%, 50.0% vs 21.2% at 24 months, p = 0.0207; Table 2). When analyzing IPSS-R subgroups, there was no significant difference in GRFS observed between treatment groups. The cumulative incidence of GvHD was similar in both groups.
Table 2. Kaplan-Meier estimates of primary and secondary endpoints*
|
GRFS, GvHD-free, relapse-free survival; GvHD, graft-versus-host disease; NS, not significant; OS, overall survival. |
|||
|
Endpoint at 24 months, % |
Fludarabine + Treosulfan |
Fludarabine + Busulfan |
p value |
|---|---|---|---|
|
Event-free survival |
|||
|
12-month |
71.4 |
60.6 |
— |
|
24-month |
68.1 |
48.2 |
0.0479792 |
|
36-month |
59.7 |
48.2 |
— |
|
Patients with events |
35.7 |
49.6 |
— |
|
Death |
21.4 |
27.0 |
— |
|
Relapse/progression |
13.1 |
19.1 |
— |
|
Primary graft failure |
1.2 |
0.9 |
— |
|
Secondary graft failure |
0 |
2.6 |
— |
|
OS |
72.8 |
53.7 |
— |
|
GRFS† |
44.5 |
33.6 |
— |
|
Cumulative incidence of relapse/progression |
10.8 |
19.6 |
— |
|
Nonrelapse mortality |
19.9 |
28.7 |
— |
|
GvHD-/relapse-/progression-free survival according to baseline blast count |
|||
|
<5% |
52.6 |
54.2 |
NS |
|
≥5–19% |
37.5 |
22.4 |
0.0324 |
|
≥10–19% |
50.0 |
21.2 |
0.0207 |
|
GvHD-/relapse-/progression-free survival according to IPSS-R risk |
|||
|
Very low/low |
49.5 |
55.9 |
NS |
|
Intermediate |
45.3 |
41.0 |
NS |
|
High/very high |
43.0 |
22.2 |
NS |
Conclusion
Stelljes et al.1 highlighted that this post-hoc analysis demonstrated that there was a trend towards better survival outcomes with treosulfan/fludarabine compared to busulfan/fludarabine, with overall rates of both acute and chronic GvHD being comparable between the treatment groups. The authors state that the results confirm the curative benefit of allo-HSCT, with RIC regimens enabling allo-HSCT to be considered in elderly patients with MDS who may have other comorbidities. The authors also conclude that although promising survival rates are seen following transplantation, more needs to be done to optimize the treatment for these patients.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

