All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Phase I results of venetoclax combined with azacitidine in high-risk MDS or CMML
During the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition, Alexandre Bazinet1 presented phase I results of the combination of venetoclax and 5-azacitidine in treatment-naïve or relapsed/refractory (R/R) high-risk myelodysplastic syndromes (MDS) or chronic myelomonocytic leukemia (CMML).1
Hypomethylating agents (HMAs), such as azacitidine, have produced modest response rates (6–34%) with limited duration of response (median, 8–10 months) in high-risk MDS, and failure on hypomethylating agents is associated with poor prognosis (overall survival, 4.3–5.6 months). Therapeutic options are limited. Pre-clinical data have shown that high-risk MDS cells survive through the action of anti-apoptotic proteins B-cell lymphoma-2 (BCL-2) and myeloid-cell leukemia-1 (MCL-1). Exposure to azacitidine shifts the balance towards BCL-2 action, making MDS cells more sensitive to BCL-2 inhibition, suggesting a synergy between venetoclax and azacitidine.
Study design
- Single center, phase I/II study (NCT04160052)
- Primary objectives include safety tolerability (phase I) and recommended phase II dose
- Eligibility criteria:
- HMA-naïve, high-risk MDS defined as intermediate-2 or high risk by International Prognostic Scoring System (IPSS) (Cohort A: HMA naïve)
- R/R MDS following ≥4 cycles of HMA (Cohort B: HMA failure)
- CMML
- ≥18 years of age
- Bone marrow blasts of 5–19%
- Exclusion criteria:
- Low-risk MDS
- Prior exposure to BCL-2 inhibitors
Figure 1 depicts the phase I dose escalation design.
Figure 1. Phase I dose escalation (3 + 3 design)*
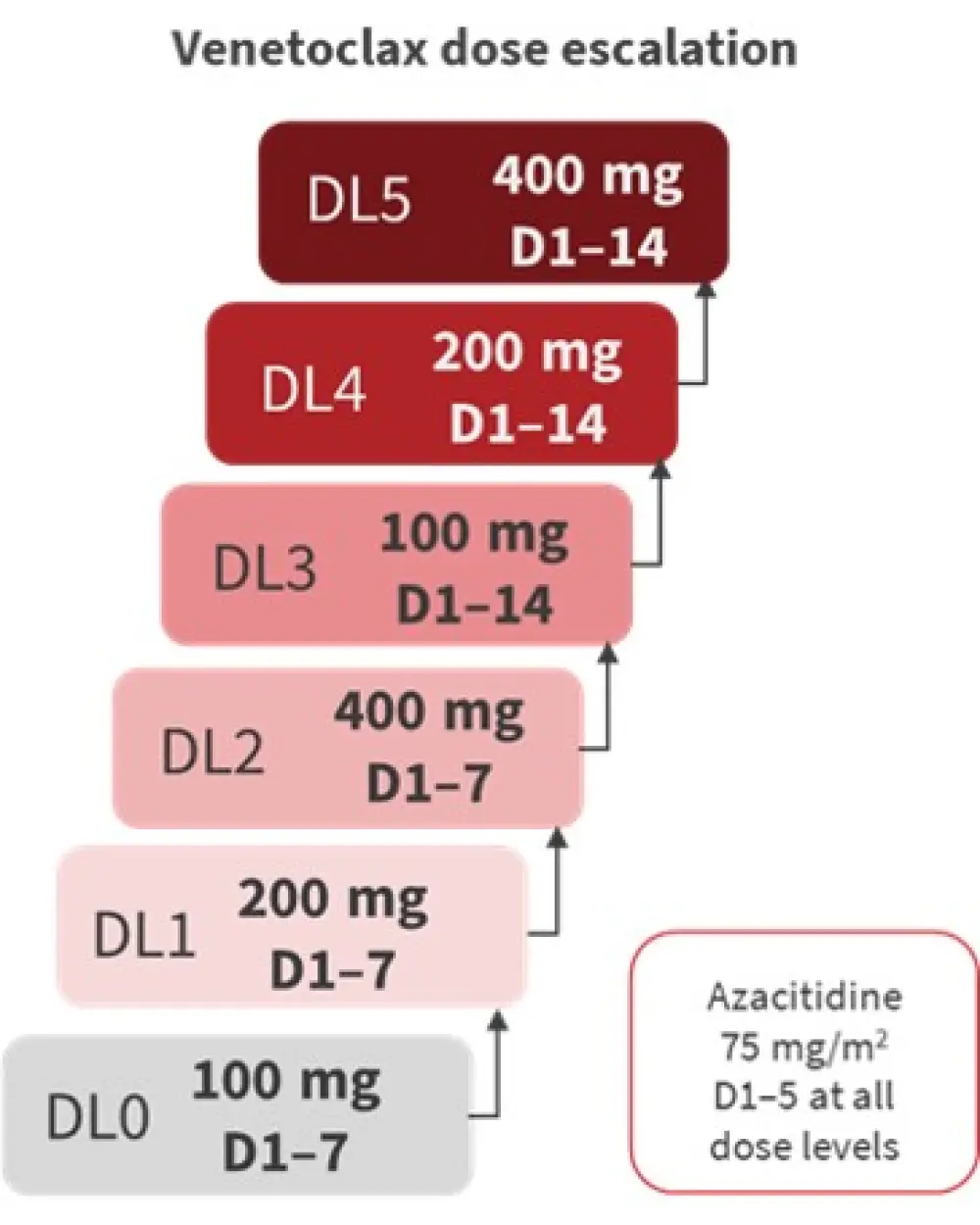
D, Day; DL, dose level.
*Adapted from Bazinet, et al.1
Results
Twenty patients were enrolled in the phase I study. The median age was 68.5 years (range, 58–84). Cohort A included 14 patients, and cohort B included six patients. Patient characteristics are summarized in Table 1.
Table 1. Baseline characteristics by cohort*
|
BM, bone marrow; CMML, chronic myelomonocytic leukemia; EB, excess blast; HMA, hypomethylating agent; IPSS, International Prognostic Scoring System; MDS, myelodysplastic syndrome; R/R, relapsed/refractory. |
||
|
Characteristic, % unless stated otherwise |
Cohort A: |
Cohort B: |
|---|---|---|
|
Median age, years (range) |
68.5 (58–83) |
67 (59–84) |
|
Diagnosis |
|
|
|
MDS-EB1† |
21 |
33 |
|
MDS-EB2‡ |
57 |
33 |
|
CMML |
21 |
33 |
|
Karyotype by IPSS |
|
|
|
Good |
57 |
17 |
|
Intermediate |
14 |
17 |
|
Poor |
29 |
67 |
|
IPSS risk category |
|
|
|
Intermediate-2 |
79 |
67 |
|
High-risk |
21 |
33 |
|
Therapy-related MDS |
21 |
33 |
|
BM blasts (range) |
12 (6–19) |
10.5 (7–17) |
The most common mutations identified in the baseline mutational profile by next-generation sequencing were TET2, ASXL1, SRSF2, and TP53 mutations. Six patients had TP53 mutations with a median variant allele frequency (VAF) of 52.3% (range, 2.4–84.4), and all had complex karyotypes.
Safety
The most common Grade 3–4 adverse events included infection (n = 9; mainly in the lungs), thrombocytopenia (n = 6), neutropenia (n = 5), and neutropenic fever (n = 2), which were expected with this combination. Three patients died due to sepsis; 4-week and 8-week mortality was 5% and 10%, respectively.
Median days to Cycle 2 was 35.5 days (range, 28–76), indicating a long time to count recovery. There was one dose-limiting toxicity, namely BM aplasia, in DL2 (400 mg, Days 1–7).
In terms of hematologic recovery, neutrophil, platelet, and hemoglobin recovery were found to be delayed or suboptimal for a large proportion of patients, suggesting continuous myelosuppression by venetoclax.
Efficacy
Seventeen patients were eligible for this analysis, and overall response rates were high, as shown in Figure 2. Responses were mainly marrow complete remission and were observed across different dose levels. Patients achieved a response after the first cycle of treatment, with a median duration of response of about 6 months (5.9 months in Cohort A; 5.8 months in Cohort B). In patients with baseline genetic abnormality, cytogenetic response rate was 22.2%.
Figure 2. Response rates*
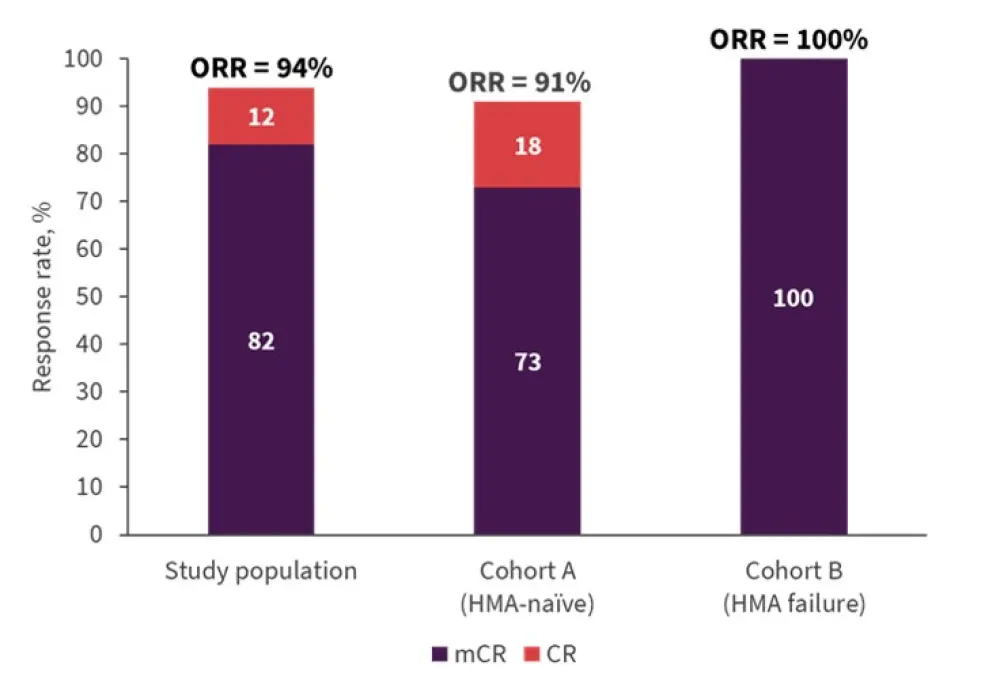
*Adapted from Bazinet, et al.1
CR, complete remission; HMA, hypomethylating agent, mCR, marrow CR; ORR, overall response rate.
Median duration of follow-up was 14.8 months.
- Median overall survival was not reached in cohort A versus 10.5 months in cohort B (p = 0.17).
- Median progression-free survival was 5.7 months and 6.8 months in cohort A and B, respectively (p = 0.56).
Reasons for study withdraws were progression to acute myeloid leukemia, recurrence of MDS/CMML, need for stem cell transplantation, and death.
Conclusion
This study indicates that the combination of azacitidine and venetoclax is tolerable in older patients with high-risk MDS/CMML; however, the occurrence of myelosuppression and infections as well as delayed count recovery were high. Dose escalation is currently ongoing to determine recommended phase II dose. In terms of preliminary efficacy, overall response rates were high, regardless of exposure to HMA; responses were mostly marrow complete remission and achieved after a median of one cycle. Median overall survival was not reached in Cohort A versus 10.5 months in cohort B, which compares favorably to previous experience. A dose expansion phase is planned to include 80 patients (n = 40 in each cohort).
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

