All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Outcomes of patients with CMML treated with hypomethylating agents
Hypomethylating agents (HMAs) currently remain the only approved drugs for the management of chronic myelomonocytic leukemia (CMML), with prospective, randomized controlled trials challenging to perform given the rarity of this aggressive neoplasm. There is therefore little clinical data and real-world evidence available to guide optimal treatment options, particularly within patient subgroups.
To garner more knowledge on therapeutic approaches, Lisa Pleyer and colleagues conducted an international retrospective cohort study in patients with CMML, and compared outcomes between those treated with HMAs, hydroxyurea, and intensive chemotherapy. Their findings have recently been published in The Lancet Haematology1 and are summarized below.
Study design and patient characteristics
Data from non-selected, consecutive patients treated for CMML were provided by 38 centers in the USA and Europe between November 30, 2017 and January 5, 2019. Patients with acute myeloid leukemia (AML; ≥ 20 blasts in bone marrow or peripheral blood) were not eligible for inclusion. Baseline characteristics for the 949 patients with CMML who were included are shown in Table 1. Subgroup stratification was performed according to first-line treatment and the following clinical parameters:
- Blast count < 10% vs ≥ 10%
- CMML-specific Prognostic Scoring System (CPSS) lower risk (lower- and intermediate-1 risk) vs higher risk (higher- and intermediate-2 risk)
- World Health Organization (WHO) subtype
Table 1. Patient characteristics (adapted from Plever et al.1)
|
AML, acute myeloid leukemia; CMML, chronic myelomonocytic leukemia; CPSS, CMML-specific Prognostic Scoring System; IQR, interquartile range; OS, overall survival; TTAML, time to transformation to AML; TTNT, time to next treatment. |
|
|
Characteristic |
N = 949 |
|---|---|
|
Median age, years (range) |
72.0 (65.2–77.9) |
|
Male, % |
66 |
|
Diagnosis, % |
|
|
Higher-risk CPSS, % |
56 |
|
Transformed to AML, % |
33 |
|
Median follow-up, months (IQR) |
|
|
Median time to start of first-line treatment, months (IQR) |
2.0 (0.3–8.5) |
|
Number of treatments received, % |
|
|
First-line treatment, n (%) |
|
|
Median OS from start of first-line treatment, months (IQR) |
18.5 (16.7–20.0) |
|
Median TTNT from start of first-line treatment, months (IQR) |
8.8 (8.0–10.1) |
|
Median TTAML from start of first-line treatment, months (IQR) |
16.4 (14.5–18.3) |
Results
Overall, 551 (58%) patients were treated with HMAs and 398 (42%) patients with other therapies. As shown in Figure 1, patients treated with HMAs had a longer overall survival (OS) compared to patients treated with non-HMAs, and this was also the case after multivariate adjustment for age, sex, CPSS risk, and platelet count. Stratification revealed a significant survival benefit with HMA treatment compared to non-HMA treatment for patients with myelodysplastic CMML and ≥ 10% blasts, and for patients with myeloproliferative CMML and either < 10% or ≥ 10% blasts. This benefit remained when all patients with myeloproliferative CMML were analyzed together, both unadjusted and after multivariate adjustment. For patients with myelodysplastic CMML and < 10% blasts, OS was similar regardless of therapy type.
HMA treatment was also not associated with a difference in OS for patients with lower-risk CPSS or WHO subtype CMML-0 (cases with 2% blasts in the blood and 5% blasts in the bone marrow), however within the subgroup of patients with high-risk CPSS or CMML-1/2 (cases with > 2% blasts in the blood and/or > 5% blasts in the bone marrow), HMA use conferred a survival benefit compared to those who had never received HMA treatment (Figure 1).
Figure 1. Overall survival for patients with CMML treated with HMAs and non-HMAs, stratified by proportion of blasts, CPSS risk, and WHO subtype1
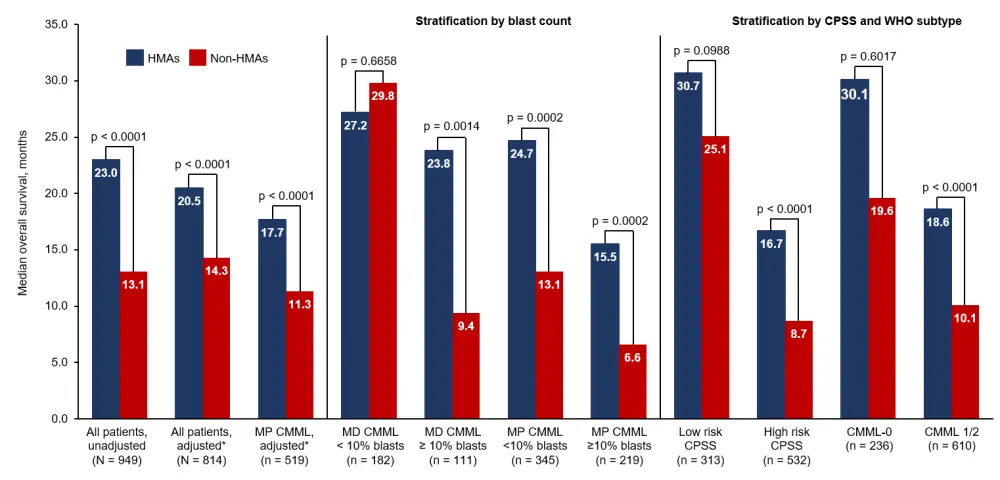
CMML, chronic myelomonocytic leukemia; CPSS, CMML-specific Prognostic Scoring System; HMA, hypomethylating agent; MD, myelodysplastic; MP, myeloproliferative; WHO, World Health Organization.
*Multivariate adjustment for age, sex, CPSS, and platelet count.
Stratification by first-line treatment
Table 2 shows outcomes for patients with CMML when grouped according to treatment with HMAs, hydroxyurea, and intensive chemotherapy. Key findings were:
- Improved mortality at 30 and 60 days with HMAs vs intensive chemotherapy, and at 60 days for HMAs vs hydroxyurea.
- Prolonged OS, time to next treatment (TTNT), and time to transformation to AML (TTAML) in patients treated with HMAs compared to hydroxyurea and intensive chemotherapy both in unadjusted analysis and following multivariate adjustment for age, sex, CPSS risk, and platelet count.
Table 2. Outcomes by treatment type1
|
CI, confidence interval; HMA, hypomethylating agent; OS, overall survival; TTAML, time to transformation to acute myeloid leukemia; TTNT, time to next treatment. |
||||
|
Outcome |
HMA |
Hydroxyurea |
Intensive chemotherapy |
p value |
|---|---|---|---|---|
|
Mortality, % |
|
|
|
|
|
Median OS, months (95% CI) |
|
|
|
|
|
Median TTNT, months (95% CI) |
|
|
|
|
|
Median TTAML, months (95% CI) |
|
|
|
|
In patients with myeloproliferative CMML, multivariate analysis revealed:
- Treatment with HMAs was associated with improved median OS (17.6 months) compared to both hydroxyurea (12.6 months; HR, 1.38; 95% CI, 1.12–1.70; p = 0.0027) and intensive chemotherapy (12.3 months; HR, 1.44; 95% CI, 1.02–2.03; p = 0.040).
- Median TTNT was greater in patients treated with HMAs (10.0 months) than hydroxyurea (5.7 months; HR, 1.64; 95% CI, 1.34–2.00; p < 0.0001) and intensive chemotherapy (6.0 months; HR, 1.59; 95% CI, 1.14–2.22; p = 0.0063).
- Median TTAML was also longer for patients treated with HMAs (16.9 months) compared to hydroxyurea (10.2 months; HR, 1.57; 95% CI, 1.24–1.98; p = 0.0001) and intensive chemotherapy (10.7 months; HR, 1.51; 95% CI, 0.98–2.31; p = 0.061).
Multivariate analysis also showed a significant survival, TTNT, and TTAML benefit for patients with ≥ 10% blasts or WHO subtype CMML-1/2 who received HMAs compared to those who received hydroxyurea or intensive chemotherapy. However, OS was similar across treatment types for patients with myelodysplastic CMML, < 10% blasts, or WHO subtype CMML-0. Furthermore, patients treated with HMAs had:
- Decreased risk of death vs hydroxyurea (HR, 1.32; 95% CI, 1.11–1.52; p = 0.0020) and vs intensive chemotherapy (HR, 1.43; 95% CI, 1.07–1.91; p = 0.016).
- Reduced need for next treatment vs hydroxyurea (HR, 1.42; 95% CI, 1.20–1.68; p < 0.0001) and vs intensive chemotherapy (HR, 1.48; 95% CI, 1.12–1.95; p = 0.0057).
- Shorter TTAML vs hydroxyurea (HR, 1.45; 95% CI, 1.20–1.76; p = 0.0001) and vs intensive chemotherapy (HR, 1.57; 95% CI, 1.12–2.20; p = 0.0096).
Conclusion
This retrospective cohort study provides much needed data on survival benefits in patients with CMML treated with HMAs compared with both hydroxyurea and intensive chemotherapy. Moreover, improved outcomes were found with HMAs compared with hydroxyurea or intensive chemotherapy in subgroups of patients with higher-risk features, such as myeloproliferative CMML, blast count ≥ 10%, WHO subtype CMML-1/2, or higher-risk CPSS, whereas this benefit was not observed in patients with lower-risk features (myelodysplastic CMML with fewer than 10% blasts, WHO subtype CMML-0, or lower-risk CPSS).
Given that this study was retrospective, there are inherent limitations such as potential selection bias and investigator choice. However, the findings support current European Medicines Agency restrictions of azacitidine in myelodysplastic CMML to patients with ≥ 10% blasts and in myeloproliferative CMML with CMML-1/2, in addition to the recommendation not to use intensive chemotherapy in myeloproliferative CMML. The authors proposed that these restrictions could be amended to favor the use of HMAs in all patients with myeloproliferative CMML, regardless of blast count, rather than hydroxyurea, which is currently considered the gold standard in patients with myeloproliferative features who are ineligible for stem cell transplant.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

