All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Oral cedazuridine/decitabine offers comparable efficacy and safety to IV decitabine in MDS and CMML, with enhanced QoL opportunities
DNA methyltransferase (DNMT) inhibitors, such as decitabine, are used in the treatment of patients with myelodysplastic syndromes (MDS), chronic myelomonocytic leukemia (CMML), and patients with acute myeloid leukemia (AML) ineligible for high-intensity therapy. Multiple cycles of parenteral decitabine are generally needed for maximal clinical response. As this treatment commonly requires 5–7 days hospital attendance per cycle, an orally bioavailable DNMT inhibitor could improve quality of life, allowing for home-based treatment and reducing the burden associated with clinic/hospital visits. The oral bioavailability of decitabine is limited due to rapid inactivation by cytidine deaminase in the gastrointestinal tract and liver. Cytidine deaminase inhibition represents a viable approach to improving the oral bioavailability of DNMT inhibitors.
Garcia-Manero et al. conducted a phase II study (NCT02103478) to compare systemic decitabine exposure, demethylation activity, and safety with cedazuridine versus standard IV decitabine. The trial was carried out in adult patients with International Prognostic Scoring System intermediate-1/2 or high-risk MDS or CMML. Study results have been recently published in Blood,1 and here we summarize the key points.
Study design1
- Multicenter, open-label, randomized crossover study.
- Eligible patients were initially randomized 1:1 to receive one of two treatment regimens during the first two 28-day cycles: oral cedazuridine/decitabine daily for 5 days in Cycle 1, followed by IV decitabine daily for 5 days in Cycle 2 (sequence A); or IV decitabine in Cycle 1, followed by the oral drug in Cycle 2 (sequence B).
On study entry, one prior cycle of either decitabine or azacitidine was permitted, but no other cytotoxic chemotherapy within 2 weeks of study treatment. Patients were required to be free of graft-versus-host disease and off immunosuppressive therapy at time of enrollment.
Table 1. Study endpoints1
|
AML, acute myeloid leukemia; IV, intravenous; PK, pharmacokinetics. |
|
|
Primary endpoints |
Secondary endpoints |
|---|---|
|
Oral/IV decitabine exposure over 5 days DNA demethylation of oral cedazuridine/ decitabine vs IV decitabine from the first 2 cycles Overall response rate using International Working Group 2006 criteria |
Duration of response, transfusion independence, time to AML, and survival Other PK measurements Assessment of the safety of oral cedazuridine/decitabine vs IV decitabine in the first cycles, and of the oral drug from Cycle 3 onwards |
Patients1
- 138 patients were screened, with 86 randomized to dose confirmation (n = 52) and fixed dose combination (n = 34) cohorts, respectively.
- Of these patients, 6 did not receive any study treatment and were excluded from all analyses.
- At data cutoff, there were 67 patient discontinuations with 13 remaining on treatment.
- Baseline characteristics were generally balanced across the first two randomized treatment sequences in each of the two cohorts. Overall (N = 80) baseline characteristics were as follows:
- Median age 69.7 years (range, 32–90)
- 76% male
- 93% White, 2.5% African American, 5% other
- ECOG performance status 0, 1 and 2 (44%, 48%, and 9%, respectively).
Efficacy1
Response to treatment was seen in 48 of 80 patients (60%), including 17 (21%) with complete responses (CR; Table 2). The median duration of CR was 13.3 months (95% CI, 6.5–13.8), the time to first response and time to best response by cycle can be seen in Figure 1. Response appeared as early as the first cycle, however most responses were manifest by Cycle 3, and the best response could take up to five or more cycles. Transfusion independence was achieved in 19 of the 38 (50%) patients who were red blood cell transfusion-dependent at baseline, 19 became transfusion independent. Of the 12 patients who were platelet transfusion-dependent at baseline, 6 (50%) became transfusion independent. Median time to AML or death for the overall population treated was 12.1 months (95% CI, 5.9–not estimable). For all patients treated, the median overall survival was 18.3 months (95% CI, 9.1–not estimable). There was no clinically or statistically significant difference in the effect on global DNA methylation between oral and IV dosing.
Table 2. Analysis of best response (adapted from Garcia-Manero et al.1)
|
CR, complete response; HI, hematologic improvement; HI-E, erythroid response; HI-N, neutrophil response; HI-P, platelet response; mCR, marrow complete response; PR, partial response. *Patients are counted only once with their best response as per the table hierarchy. |
||
|
Type of response |
Phase II overall (N = 80) |
|
|---|---|---|
|
% |
95% CI |
|
|
CR |
21 |
13–32 |
|
PR |
0 |
|
|
mCR mCR with HI |
22 7 |
14–33 3–16 |
|
HI HI-E HI-N HI-P |
16 10 2 14 |
9–26 4–19 0–9 7–23 |
|
Overall response* (CR + PR + mCR + HI) |
60 |
48–71 |
|
No response |
40 |
29–52 |
Figure 1. Time to first response and time to best response by cycle (N = 80) (adapted from Garcia-Manero et al.1)
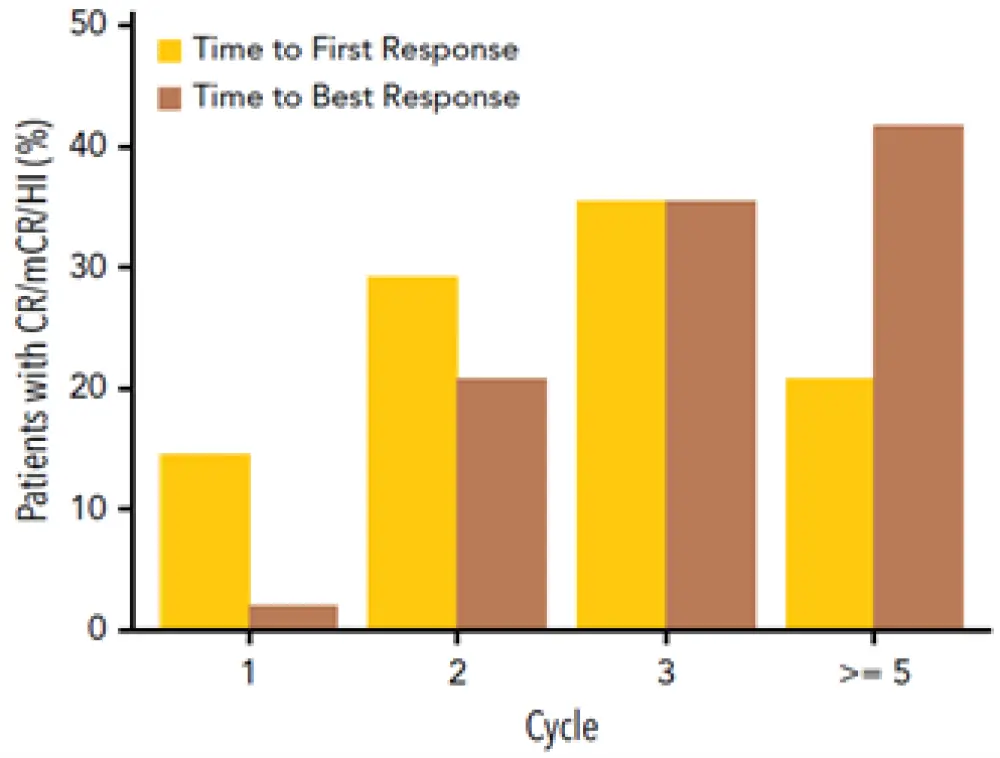
CR, complete response; mCR, marrow complete response; HI, hematologic improvement.
Safety1
There were similarities in the incidences of adverse events (AEs) during Cycles 1 and 2 between oral and IV treatment of all grades. Gastrointestinal AEs showed no notable increase with oral decitabine versus IV decitabine in the first two randomized cycles. AEs led to five discontinuations, none of which were considered related to study therapy. There were 11 deaths, including four from sepsis/septic shock and two from pneumonia, and one each from respiratory failure, cardiac arrest, sudden death, myocarditis, and small-cell lung cancer. The most common treatment emergent adverse events (TEAEs) and most common Grade ≥ 3 TEAEs for both IV decitabine and oral cedazuridine/decitabine were neutropenia and thrombocytopenia.
Conclusion
This study demonstrated that a more convenient oral route of cedazuridine/decitabine (100/35 mg) administration produced similar systemic decitabine exposure, efficacy, DNA demethylation, and safety versus decitabine 20 mg/m2 IV in the first two cycles. The benefits of a clinically equivalent oral form of decitabine treatment offers promising opportunities in the treatment of myeloid malignancies with the goal of further improving treatment outcomes whilst enhancing quality of life and minimizing treatment burden.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

