All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Lenalidomide reduces risk of transfusion dependency in lower-risk MDS: Interim results of the Sintra-REV trial
The therapeutic goal for patients with lower-risk myelodysplastic syndrome (MDS) is to improve bone marrow function and reduce the requirement for red blood cell transfusions. Lenalidomide has been shown to offer clinical benefit for lower-risk patients with 5q deletion [del(5q)] but is currently only approved for patients with transfusion dependency due to toxicity concerns. It is proposed that early treatment with lenalidomide at a low dose may prolong the time to transfusion dependency, and ultimately improve outcomes.1
The interim results of the phase III Sintra-REV study (NCT01243476; data cut-off, July 2020), investigating the efficacy and safety of early treatment with low-dose lenalidomide in patients with lower-risk MDS and del(5q), were presented by Maria Diez-Campelo during the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition.1 Here we summarize the findings.
Trial design
A phase III, multicenter, double-blind, randomized, placebo-controlled study, with the following patient inclusion criteria:
- MDS diagnosis (World Health Organization 2008)
- International prognostic scoring system (IPSS) low or intermediate-1
- No red blood cell transfusion requirements
- Anemia (hemoglobin <12 g/dL)
- Del(5q) (+/- other abnormality)
A summary of the study design is shown in Figure 1.
Figure 1. Sintra-REV trial design1
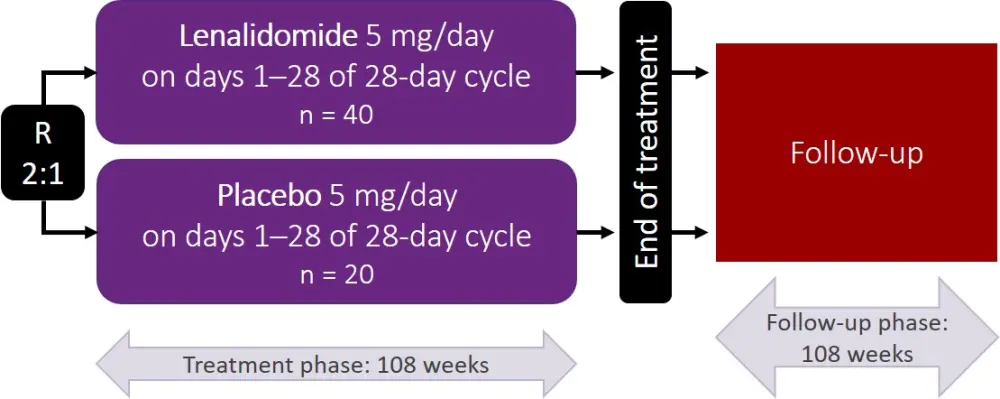
R, randomization.
Primary endpoint:
- Efficacy determined by time to disease progression and transfusion dependency
Secondary objectives included:
- Erythroid and cytogenetic response
- Safety and tolerability
- Overall survival, event-free survival, and transformation to acute myeloid leukemia
Patient characteristics and disposition
Selected patient characteristics are shown in Table 1. There were no significant differences observed between the lenalidomide and placebo arms.
Table 1. Selected patient characteristics1
|
IPSS, international prognostic scoring system. *Additional cytogenetic abnormalities: +8, t(1;13), -Y, -7 (lenalidomide arm), and del(11q), -Y (placebo arm). |
||
|
Lenalidomide |
Placebo |
|
|---|---|---|
|
Sex, female, % |
80.0 |
85.7 |
|
Median age, years |
72.2 |
71.9 |
|
IPSS low, % |
87.5 |
81.0 |
|
IPSS intermediate-1, % |
12.5 |
19.0 |
|
Isolated del(5q) abnormality, % |
90.0 |
90.5 |
|
Additional cytogenetic abnormality*, % |
10.0 |
9.5 |
|
Hemoglobin, g/dL |
9.8 |
9.8 |
|
Absolute neutrophil count, ×109/L |
2.1 |
2.2 |
|
Platelets, ×109/L |
238 |
272 |
|
Peripheral blood blasts, % |
0 |
0 |
|
Bone marrow blasts, % |
1.5 |
2.0 |
|
Time to Sintra-REV, months |
2.7 |
4.0 |
At the time of reporting, >80% of patients had completed the trial protocol. The median time on treatment was higher in the lenalidomide arm compared with the placebo arm (23.9 vs 10.6 months). Lenalidomide treatment was discontinued in 52.5% of patients, mainly due to progressive disease and need for transfusion (38%), rejection of informed consent (28.5%), or physician decision (23.8%). In the placebo arm, treatment was discontinued in 66.6% of patients; progressive disease and transfusion need accounted for 71.4% of these cases.
Efficacy
- Transfusion dependency was observed in 27.5% of patients treated with lenalidomide, compared with 57.1% of patients treated with placebo (hazard ratio, 0.338; p = 0.028).
- Time to transfusion dependency was delayed in the lenalidomide arm compared with the placebo arm (median transfusion-free survival, 6.3 years vs 2.8 years; p = 0.023).
- Lenalidomide treatment resulted in erythroid response in 72.5% of patients, and cytogenetic response in 80% of patients; of these, 87.5% achieved a complete cytogenetic response.
- Patients in the placebo arm did not achieve either erythroid or cytogenetic responses.
- Median overall survival was not reached in either treatment arm.
Safety
Low-dose lenalidomide was considered safe and well-tolerated. Grade 3–4 adverse events are summarized in Table 2. Although almost half of patients in the lenalidomide arm developed neutropenia, only one developed febrile neutropenia and there were no neutropenia-related complications requiring hospitalization.
There were nine deaths in total, none related to study treatment, and acute myeloid leukemia was diagnosed in three patients, of which two (5%) were in the lenalidomide arm and one (5%) in the placebo arm.
Table 2. Grade 3–4 adverse events1
|
AE, adverse event; DVT, deep vein thrombosis; PE, pulmonary embolism. |
||
|
|
Lenalidomide |
Placebo |
|---|---|---|
|
Non-hematologic Grade 3–4 AE, % |
||
|
Vascular (PE/DVT) |
2.6 |
0 |
|
Rash |
2.6 |
0 |
|
Second neoplasm |
5.3 |
0 |
|
Hematologic Grade 3–4 AE, % |
||
|
Anemia |
2.6 |
0 |
|
Thrombocytopenia |
5.3 |
0 |
|
Neutropenia |
46.8 |
4.8 |
|
Febrile neutropenia |
2.6 |
0 |
Conclusions
In this interim analysis of the phase III Sintra-REV trial, early treatment with low-dose lenalidomide delayed the time to and reduced the risk of transfusion dependency for patients with lower-risk, del(5q) MDS. Erythroid and cytogenetic responses were observed in 72.5% and 80% of patients, respectively, and the low-dose treatment had an acceptable safety profile. Since the follow-up data is not yet mature, the impact of this regimen on long-term outcome is still to be determined.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

