All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Iron overload screening, treatment modalities, and therapies in pediatric post-HSCT patients
The long-term clinical impact of transfusional iron overload and toxicity is not known in pediatric cancer survivors and patients who undergo hematopoietic stem cell transplantation (HSCT) for nonmalignant and malignant disorders. Recently, a variety of surveillance modalities that can be used to monitor iron overload during and after treatment in pediatric cancer and blood and marrow transplantation (BMT) survivors, were presented by Lalefar NR during the 2021 Conference of the American Society of Pediatric Hematology/Oncology (ASPHO).1
According to the recommendations made by the Children’s Oncology Group (COG) in their 2018 guideline, the following methods can be used for screening and testing iron overload:
- Ferritin screening and evaluation of iron content using T2*-based magnetic resonance imaging (MRI)
- A liver biopsy if there is excessive iron content
- Potential phlebotomy or chelation therapy for treating iron overload
Who should be monitored and how?
Post-therapy iron overload occurs due to an acute rise in free iron and decrease in iron utilization after chemo-induced erythroid hyperplasia in the bone marrow. Therefore, each patient should be assessed individually post-therapy. A total transfused volume of >100 ml/kilo, or approximately 10 transfusions, is associated with an increase in iron burden in children.
The COG 2018 guidelines for iron overload in pediatric oncology and BMT survivors, recommends the evaluation of packed red blood cell (pRBC) transfusion exposure post end-of-therapy hematopoietic recovery.
- Ferritin is used as a surrogate marker of total body iron stores. Evaluation of other serum markers (Table 1) in fasting is also recommended for iron overload evaluation.
Table 1. Serum markers to identify iron overload*
|
*Adapted from Lalefar NR, et al.1 |
|
|
Serum markers |
Iron overload |
|---|---|
|
Serum iron |
↑ |
|
Serum ferritin |
↑ (>1,000 µg/L) |
|
Transferrin iron saturation, % |
↑ (>50%) |
|
Transferrin |
↓ |
|
Total iron binding capacity |
↓ |
|
Hemoglobin |
Normal |
- Ferritin monitoring has the advantage of being repeatable, noninvasive, widely available, and inexpensive.
- However, ferritin has the disadvantage of having a nonlinear relationship with iron overload and can be confounded by inflammation because it is an acute-phase reactant.
Methods for iron-overload monitoring
Several clinical methods are used to detect presence of excessive iron in various tissues (Table 2).
Table 2. Modalities to measure iron overload†
|
BMT, blood and marrow transplantation; GvHD, graft-versus-host disease; MRI, magnetic resonance imaging. |
|||||
|
Modality |
T2* MRI |
Cardiac T2* MRI |
FerriScan® |
SQUID |
Liver biopsy |
|---|---|---|---|---|---|
|
Principle |
An indirect instrumental estimation of tissue iron concentration. |
Detects myocardial iron accumulation |
Same as T2* MRI |
Measures the interference of an exteriorly applied small, but highly constant, magnetic field by the paramagnetic liver storage iron of the patient. |
Direct quantitative measure of liver iron concentration (LIC). |
|
Normal iron concentration |
80−480 µg/g wet liver weight OR 0.48−2.8 mg/g liver dry weight |
T2* >20 ms, no cardiac iron detected. |
— |
— |
— |
|
Abnormal values/iron overload/threshold |
>7 mg/g of liver dry weight |
T2* <10 ms, severe cardiac deposition of iron |
>1,000 ng/ml |
— |
— |
|
Advantages |
Noninvasive, and repeatable. |
— |
Clinically validated in the liver. |
Direct instrumental measure of hepatic iron concentration. |
Good at excluding other liver dysfunction such as fibrosis, cirrhosis, viral hepatitis, fatty liver, and cases of BMT patients for GvHD. |
|
Disadvantages |
Expensive, low accessibility, and may require anesthesia in some cases. |
Expert handling. |
Same as T2*MRI. |
Limited availability. |
Invasive, susceptible to sampling errors, and not feasible for patients with thrombocytopenia or coagulopathy. |
Treatment
Frequently used treatments for iron overload are as follows:
- Phlebotomy
- Iron chelators
- deferasirox
- deferoxamine
Phlebotomy
- Phlebotomy is used when Hb >11 g/dl and 5 ml/kg of blood is replaced with an equal volume of saline/oral fluid
- Ferritin and iron should be measured every 3 months to check if the values are trending normal and patients are not becoming iron deficient
- Liver iron concentration (LIC) is measured every 6−12 months
- It is safe and cost-effective
- However, frequent clinical visits are required depending on the patient; patients should come in for phlebotomy every 2 to 4 weeks
Iron chelators − deferasirox
- It is a tridentate iron chelator with good bioavailability, long half-life, and suitable for once/day
- Initial dose is 3.5 mg/kg/day, which can be increased up to 7 mg/kg/day over 3−6 months depending on iron studies
- Dose-related toxicities: Skin rash, nausea, diarrhea
- Other toxicities:
- Transaminitis
- Renal toxicities because of the risk of proximal renal tubule dysfunction; therefore, it is important to measure metabolic panel and phosphorus to prevent metabolic acidosis and hypophosphatemia
- Contraindicated when the platelet count is <50 k, because of high risk of gastrointestinal (GI) hemorrhage that can be seen with these patients
Iron chelation − deferoxamine
- Hexadentate iron chelator binds one-to-one specifically with a ferric iron
- It is administered by infusion with an ambulatory pump, either subcutaneously or intravenously
- Treatment begins at a lower dose (20 mg/kg) for 8 hours/3 nights/week
- Toxicity comes with higher doses (>30 mg/kg)
- Toxicities that can be seen are the following: high-frequency sensorineural hearing loss, retinal, metaphyseal/spinal, or growth retardation
- Depending on the severity, lowering of dose, or stopping the treatment is recommended
Phlebotomy vs oral chelation
Both methods can result in the reduction of iron burden in patients. However, it was observed that LIC reductions were greater with deferasirox than with phlebotomy for patients with a baseline serum ferritin of 1,000 ng/ml or higher (−8.1 ± 1.5 vs −3.5 ± 5.7 mg Fe/g dw; P = 0.048). Serum ferritin and nontransferrin-bound iron also decreased significantly.2
Surveillance and treatment algorithm
The treatment regimen will depend on the condition of the patient after BMT. Figure 1 summarizes the suggested treatment algorithm for posttransplant pediatric patients with iron overload.
Figure 1. Surveillance and treatment algorithm*
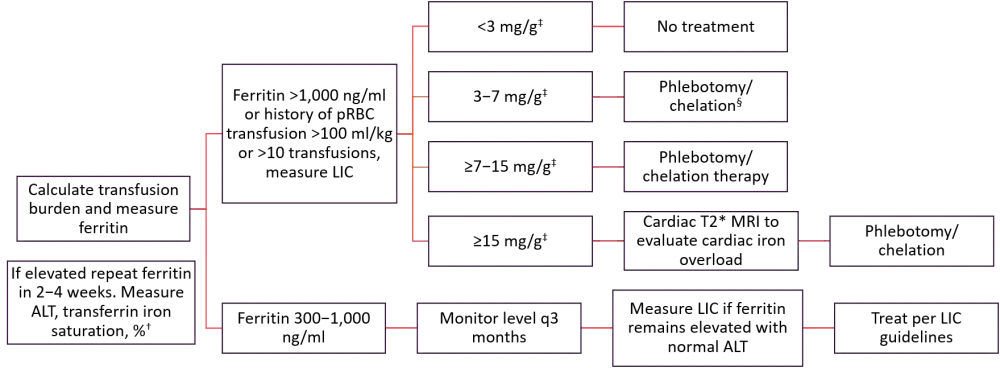
ALT, alanine aminotransferase; LIC, liver iron concentration; MRI, magnetic resonance imaging; pRBCs, packed red blood cells.
*Adapted from Lalefar et al.1
†Hb should be normal post-chemotherapy. Blood and marrow transplantation (BMT) patients should wait >100 days.
‡Liver dry weight.
§Post-pubertal adolescent/young adults, chronic GvHD, hepatitis C, history of liver fibrosis.
Implication of practice guidelines
According to a survey involving 55 participating centers carried out by the Pediatric Blood and Marrow Transplant Consortium (PBMTC) transplant centers in North America, 48% of the centers had practice guidelines for assessment and management of iron load, where ferritin was used as a surveillance modality in 100%, FerriScan® in 90.2%, liver biopsy in 56.1%, and SQUID in 12.2%.
It was observed that oral chelation was the most popular modality, with an 87.5% score, followed by phlebotomy (82.5%), intravenous chelation (67.5%), a combination of chelation and phlebotomy (52.5%), and RBC pheresis (17.5%).
Conclusion
Iron overload is common in pediatric patients after HSCT and is associated with hepatic, cardiac, and endocrine dysfunction. Therefore, monitoring the serum iron and ferritin levels using the recommended modalities and tailored treatment as per condition of the patient, are necessary.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

