All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Impact of type of blood cancer on the outcome following stem cell transplantation
Hematopoietic stem cell transplant (HSCT) is an effective treatment for many forms of blood cancer, including acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS). However, the impact of cancer type on HSCT outcome has not been examined between patients with MDS and AML. In a study by Masamitsu Yanada and colleagues, the effect of HSCT was examined in three groups of patients.1
Study design
This study looked at patients aged ≥16 years and included patients with
- de novo AML (except acute promyelocytic leukemia);
- AML evolved from MDS; or
- MDS with excess blasts (MDS-EB).
Patients with MDS were eligible if they had undergone hematopoietic cell transplant between 2008 and 2017 from a matched unrelated donor, matched related, a haploidentical donor, or umbilical cord unit. Patients were excluded if they had been diagnosed with therapy-related myeloid neoplasms.
Results
Baseline characteristics
The study included 4,520 patients and their baseline characteristics are shown in Table 1. Patients with de novo AML constituted the largest group at 73% and the median age was the lowest for this subgroup, at 49 years. Patients with AML evolved from MDS had the oldest median age but constituted only 4.6% of the whole group. The median follow-up was 4.3 years for patients who survived (interquartile range, 2.3−6.6 years). Patients with de novo AML had a lower Eastern Cooperative Group performance status and hematopoietic cell transplantation-comorbidity index compared with either group of patients with MDS.
Table 1. Baseline patient characteristics*
|
AML, acute myeloid leukemia; GvHD, graft-versus-host disease; HCT-CI, hematopoietic cell transplantation-specific comorbidity index; MDS, myelodysplastic syndromes; MDS-EB, MDS with excess blasts. |
||||
|
Characteristic, % (unless otherwise stated) |
De novo AML |
AML evolved from MDS |
MDS-EB |
p value |
|---|---|---|---|---|
|
Age |
|
|
|
<0.001 |
|
Median, years (range) |
49 (16−85) |
60 (24−77) |
58 (16−75) |
|
|
16−49 years |
52 |
20 |
25 |
|
|
≥50 years |
48 |
80 |
75 |
|
|
Sex |
|
|
|
<0.001 |
|
Female |
44 |
36 |
34 |
|
|
Performance status |
|
|
|
<0.001 |
|
0−1 |
97 |
93 |
92 |
|
|
2−4 |
3 |
7 |
8 |
|
|
HCT-CI |
|
|
|
<0.001 |
|
0 |
65 |
51 |
55 |
|
|
1−2 |
25 |
27 |
24 |
|
|
≥3 |
11 |
22 |
21 |
|
|
Cytogenetic risk |
|
|
|
<0.001 |
|
Favorable |
15 |
0 |
0 |
|
|
Intermediate |
64 |
73 |
60 |
|
|
Poor |
15 |
24 |
37 |
|
|
Unevaluable |
7 |
3 |
3 |
|
|
Donor type |
|
|
|
<0.001 |
|
Matched related |
35 |
25 |
29 |
|
|
Matched unrelated |
23 |
23 |
21 |
|
|
Umbilical cord blood |
35 |
44 |
43 |
|
|
Haploidentical related |
6 |
8 |
7 |
|
|
Conditioning |
|
|
|
<0.001 |
|
Myeloablative |
73 |
52 |
58 |
|
|
Reduced-intensity |
27 |
48 |
42 |
|
|
GvHD prophylaxis |
|
|
|
0.039 |
|
Cyclosporine-based |
43 |
36 |
39 |
|
|
Tacrolimus-based |
57 |
64 |
61 |
|
Cytogenetic risk was analyzed for the different cohorts and was highest in MDS-EB at 37% of patients with poor risk scores.
Compared with the patients with de novo AML, patients with AML evolved from MDS had a significantly decreased chance of neutrophil engraftment (p = 0.033). Patients with MDS-EB had a significantly decreased chance of both neutrophil and platelet engraftment (both p < 0.001). With respect to risk of acute graft-versus-host disease (GvHD), patients with MDS-EB were recorded to have an increased risk of Grades III and IV compared with patients with de novo AML.
Survival outcomes
Overall survival at 5 years is shown in Figure 1. There was a significant difference between patients with de novo AML compared with patients with MDS-EB (p < 0.001).
Figure 1. OS, relapse incidence, and NRM at 5 years for each of the three groups of patients*
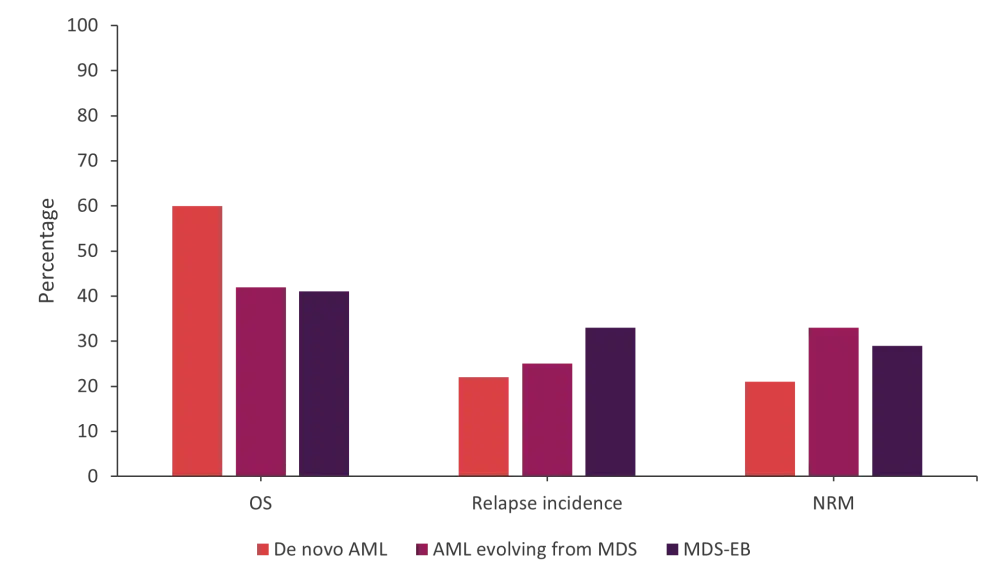
AML, acute myeloid leukemia; MDS, myelodysplastic syndromes; MDS-EB, MDS with excess blasts; NRM, non-relapse mortality; OS, overall survival.
*Data from Yanada, et al.1
Overall mortality was significantly increased in patients with AML that evolved from MDS and for patients with MDS-EB compared with patients with de novo AML (p < 0.030; Table 2). There was no significant difference between the two groups of patients with MDS-EB and AML evolved from MDS in terms of overall mortality (p = 0.804).
The incidence of relapse post HSCT at 5 years was 22% (95% confidence interval [CI], 20–23%) for patients with de novo AML compared with 33% (95% CI, 30–36%) for patients with MDS-EB (p < 0.001; Figure 1).
The incidence of relapse was significantly increased for MDS-EB compared with de novo AML but not for the other group of patients. There was no significant difference between relapse incidence in the MDS-EB group compared with the patients with AML evolved from MDS (p = 0.108).
Table 2. Overall mortality, relapse, and non-relapse mortality*
|
AML, acute myeloid leukemia; CI, confidence interval; GvHD, graft-versus-host disease; HCT-CI, hematopoietic cell transplantation-specific comorbidity index; HR, hazard ratio; MDS, myelodysplastic syndromes; MDS-EB, MDS with excess blasts. |
||||||
|
Variable |
Overall mortality |
Relapse |
Non-relapse mortality |
|||
|---|---|---|---|---|---|---|
|
HR |
p value |
HR |
p value |
HR |
p value |
|
|
Disease |
|
|
|
|
|
|
|
De novo AML |
1.00 |
|
1.00 |
|
1.00 |
|
|
AML evolved from MDS |
1.30 (1.07−1.59) |
0.010 |
1.12 (0.83−1.50) |
0.456 |
1.33 (1.03−1.72) |
0.030 |
|
MDS-EB |
1.34 (1.20−1.49) |
<0.001 |
1.43 (1.24−1.65) |
<0.001 |
1.10 (0.94−1.29) |
0.230 |
|
Age, years |
|
|
|
|
|
|
|
<50 |
1.00 |
|
1.00 |
|
1.00 |
|
|
≥50 |
1.62 (1.46−1.81) |
<0.001 |
1.07 (0.91−1.25) |
0.133 |
1.70 (1.45−1.98) |
<0.001 |
|
Sex |
|
|
|
|
|
|
|
Male |
1.32 (1.20−1.46) |
<0.001 |
0.99 (0.87−1.13) |
0.910 |
1.42 (1.24−1.62) |
<0.001 |
|
Female |
1.00 |
|
1.00 |
|
1.00 |
|
|
Performance status |
|
|
|
|
|
|
|
0−1 |
1.00 |
|
1.00 |
|
1.00 |
|
|
≥2 |
1.74 (1.45−1.81) |
<0.001 |
1.24 (0.94−1.65) |
0.133 |
1.52 (1.17−1.98) |
0.002 |
|
HCT-CI |
|
|
|
|
|
|
|
0 |
1.00 |
|
1.00 |
|
1.00 |
|
|
1−2 |
1.16 (1.04-1.30) |
0.006 |
1.01 (0.87−1.17) |
0.935 |
1.25 (1.08−1.45) |
0.003 |
|
≥3 |
1.40 (1.23−1.59) |
<0.001 |
0.98 (0.81−1.18) |
0.823 |
1.50 (1.26−1.78) |
<0.001 |
|
Cytogenetic risk |
|
|
|
|
|
|
|
Favorable |
0.85 (0.71−1.01) |
0.006 |
0.88 (0.69−1.12) |
0.288 |
0.87 (0.69−1.10) |
0.239 |
|
Intermediate |
1.00 |
|
1.00 |
|
1.00 |
|
|
Poor |
1.68 (1.51−1.87) |
<0.001 |
2.20 (1.92−2.52) |
<0.001 |
0.97 (0.83−1.14) |
0.740 |
|
Unevaluable |
1.21 (0.99–1.48) |
0.068 |
1.40 (1.08−1.81) |
0.011 |
0.92 (0.68−1.22) |
0.539 |
|
Donor type |
|
|
|
|
|
|
|
Matched related |
1.00 |
|
1.00 |
|
1.00 |
|
|
Matched unrelated |
1.01 (0.87−1.17) |
0.909 |
0.69 (0.57−0.84) |
<0.001 |
1.28 (1.04−1.56) |
0.017 |
|
Umbilical cord blood |
1.29 (1.14−1.46) |
<0.001 |
0.68 (0.58−0.80) |
<0.001 |
1.71 (1.44−2.03) |
<0.001 |
|
Haploidentical related |
1.29 (1.04−1.60) |
0.021 |
0.81 (0.61−1.07) |
0.136 |
1.44 (1.06−1.97) |
0.020 |
The 5-year incidence of non-relapse mortality (NRM) was 21% (95% CI, 20–23%) for patients with de novo AML compared with 29% (95% CI, 26–32%) for patients with MDS-EB (p < 0.001).
The risk of NRM for patients with AML evolved from MDS was significantly increased compared with patients with de novo AML. However, when patients with MDS-EB were compared with patients with de novo AML, the difference in risk of NRM was not found to be significant. In addition, the difference in risk between the two patient groups, MDS-EB and AML evolved from MDS, was not significant (p = 0.171).
Causes of death
In this study, a total of 1,917 patients died: 37% of the patients with de novo AML, 55% of the patients with AML evolving from MDS, and 57% of the patients with MDS-EB. The main causes of death for the patients with de novo AML were
- primary disease, 37%;
- infection, 18%; and
- GvHD, 8%.
In the case of patients with MDS-EB, the causes of death were similar, with 33% of death from primary disease, 24% from infection, and 8% from GvHD. For patients with AML evolving from MDS, the leading causes of death were similar, with primary disease causing 30% of deaths, followed by infection (22%) and hemorrhage (5%).
MDS-EB subset analysis
The response to pre-transplant therapy and disease subtype was also assessed for patients with MDS-EB. Of the 994 patients with MDS-EB, 43.2% had a complete response or partial response (PR), while 21.5% achieved less than a PR and the rest received no treatment prior to transplantation.
Two disease subtypes were recorded (RAEDB-1 and RAEB-2), but these were found to not be significantly associated with post-transplant outcomes. On the other hand, pre-transplant treatment response was significant associated with a difference. Patients achieving less than PR demonstrated a significantly greater risk of relapse (p = 0.006) and overall mortality (p < 0.001). No difference was seen between untreated patients and patients achieving either complete response or PR. Poor cytogenetics was also significantly associated with an increased risk of relapse and overall mortality (both p < 0.001).
Conclusion
The study showed that survival outcomes were different between disease groups. Patients with de novo AML showed improved overall survival compared with the other two patient groups, along with a decreased risk of relapse and 5-year incidence of NRM. To improve outcomes following HSCT, this study points to improving relapse incidence for patients with MDS-EB, while for patients with AML evolving from MDS more needs to be done to reduce NRM.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

