All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Health-related quality of life in patients with MDS
Patients with myelodysplastic syndromes (MDS) often experience symptoms such as cytogenetic abnormalities, dysplastic hematopoiesis, cytopenias, fatigue, dyspnea, and pain that can impact their health-related quality of life (HRQoL).1,2 Treatment is often focused on mitigating these symptoms, particularly in patients with lower-risk MDS.2,3 Recently, HRQoL has been identified as one of the most relevant patient-reported outcomes and can be an independent prognostic predictor in patients with MDS.1,2
HRQoL assessment in patients with MDS lacks MDS-specific measures.1 Furthermore, most HRQoL assessments carried out recently have been within clinical trials and comparative effectiveness studies, which can be limited by specific patient populations and time points.2 Therefore, using disease-specific HRQoL metrics can be essential to incorporating patient feedback into MDS research.1
Several recent studies have addressed the need of HRQoL assessment in patients with MDS. Below, we summarize the key findings from the recent articles by Efficace et al.1 on clinical utility and validation of the subscales of the QUALMS from the MDS-RIGHT project published in Cancer Medicine, Stojkov et al.2 on determinants of low HRQoL in patients with MDS published in Blood Advances, and Buckstein et al.3 on the burden of red blood cell transfusions in patients with lower-risk MDS and ring sideroblasts published in Leukemia & Lymphoma.
Clinical utility and validation of subscales of the QUALMS1
Study design and patient characteristics
This study was part of the MDS-RIGHT project within the European MDS registry (EUMDS). The Quality of Life in Myelodysplasia Scale (QUALMS) has been applied in centers in the Netherlands, the United Kingdom, Israel, and Austria since January 2017. The QUALMS is administered at study entry, and at 6, 12, 18, and 24 months. The QUALMS includes 33 items that are used for scoring and 5 individual “opt-out” questions that are not scored with other items. It includes three subscales, physical burden (QUALMS-P; 14 items), emotional burden (QUALMS-E; 11 items), and benefit finding (QUALMS-BF; 3 items). A score ranging from 1 to 100 is calculated, with a higher score indicating a better HRQoL. This analysis included 270 patients with a median age of 74.0 years (interquartile range [IQR], 68.0–80.0).
Key findings
All scores had similar distribution and no floor or ceiling effects were observed (Table 1).
Table 1. Distribution characteristics of the QUALMS*
|
QUALMS, Quality of Life in Myelodysplasia Scale; QUALMS-BF, benefit finding QUALMS; QUALMS‑E, emotional burden QUALMS; QUALMS-P, physical burden QUALMS; SD, standard deviation. |
|||||||
|
Scale |
Mean |
SD |
Median |
Min |
Max |
Skew |
Kurtosis |
|---|---|---|---|---|---|---|---|
|
QUALMS-P |
63.10 |
21.80 |
64.29 |
7.14 |
100.00 |
−0.19 |
−0.81 |
|
QUALMS-E |
69.71 |
19.59 |
70.45 |
4.55 |
100.00 |
−0.50 |
−0.09 |
|
QUALMS-BF |
50.38 |
25.58 |
50.00 |
0.00 |
100.00 |
−0.22 |
−0.47 |
|
QUALMS total |
66.22 |
16.31 |
68.18 |
15.15 |
96.97 |
−0.48 |
−0.18 |
Cronbach’s alpha coefficients across 6 (n = 146), 12 (n = 99), 18 (n = 71), and 24 months (n = 36) revealed the QUALMS-P scale had the highest coefficients across each assessment (Figure 1).
Figure 1. Cronbach’s alpha coefficients of the QUALMS over time*
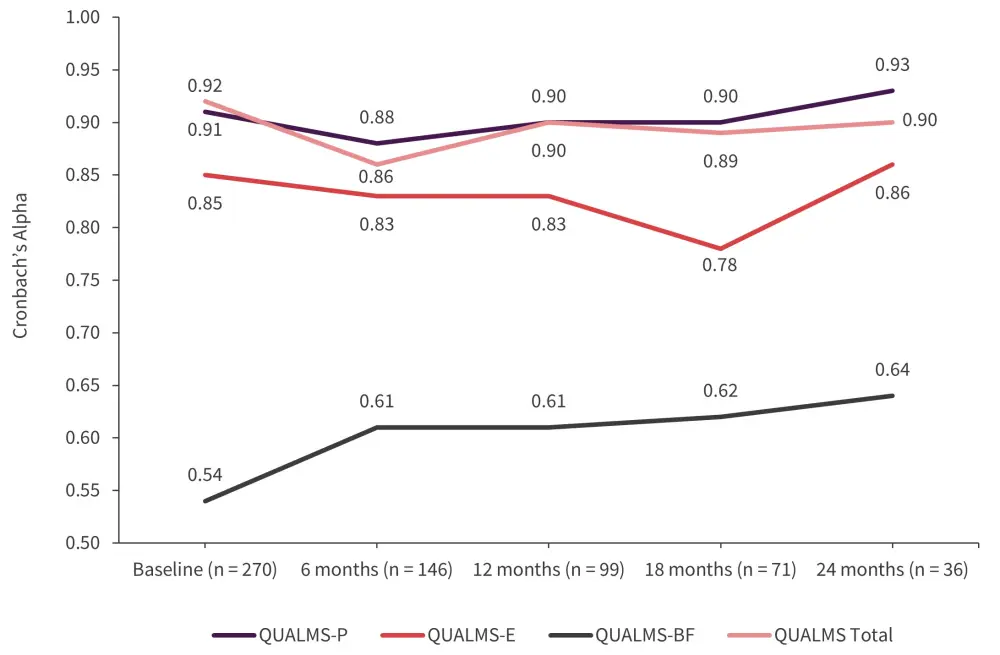
QUALMS, Quality of Life in Myelodysplasia Scale; QUALMS-BF, benefit finding QUALMS; QUALMS-E, emotional burden QUALMS; QUALMS-P, physical burden QUALMS.
*Adapted from Efficace, et al.1
- Confirmatory factor analysis supported the underlying scale structure of the QUALMS.
- The Comparative Fit Index (0.93), the Tucker-Lewis Index (0.93), and the Root Mean Squared Error of Approximation (0.07) fit indices indicated an acceptable model fit.
- There were strong positive Spearman correlation coefficients between the QUALMS total and the QUALMS-P (r = 0.92; p < 0.001) and the QUALMS-E (r = 0.87; p < 0.001) and a weak negative correlation between QUALMS total and QUALMS-BF (r = −0.15; p = 0.034).
- QUALMS-P, QUALMS-E, and the QUALMS total had moderate negative correlations with the European Quality of Life 5 Dimensions 3 Level Version (EQ-5D-3L) scales (range r: −0.26 to −0.67; p < 0.001) and moderate positive correlations with the EQ visual analog scale (VAS) (range r: 0.41–0.60; p < 0.001).
- QUALMS-BF did not show a significant correlation with any EQ-5D-3L scales and the EQ VAS.
- Patients with a low MDS-comorbidity index had better QUALMS-P (p = 0.006), QUALMS-E (p = 0.014), and QUALMS total (p = 0.006) scores than patients with an intermediate or high MDS-comorbidity index score.
- Patients with Karnofsky performance status (KPS) ≥90 had better QUALMS-P (p < 0.001), QUALMS-E (p = 0.001), and QUALMS total (p < 0.001) scores compared with patients with a KPS <90.
- Patients with anemia had worse QUALMS-P (p < 0.001), QUALMS-E (p = 0.010), and QUALMS total (p = 0.001) scores than patients without anemia.
- Transfusion-dependent patients had worse QUALMS-P (p < 0.001), QUALMS-E (p = 0.002), and QUALMS total (p < 0.001) scores than patients who were not.
- QUALMS total and the subscales mean scores changed in line with patient hemoglobin (Hb) levels in patients with a Hb level <11 g/dL at baseline who showed an improvement in their Hb levels ≥1.5 g/dL.
Determinants of low HRQoL in patients with MDS2
Study design and patient characteristics
This study used patient data from the EUMDS registry at baseline and at the 6- and 12-month visits. The EQ-5D-3L instrument was used to assess HRQoL, which contains two sections, a descriptive system and a VAS. Patients rank their health state as either no problems, some problems, or extreme problems in terms of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, which is used to calculate the EQ-5D index. The VAS allows patients to apply a score from 0 to 100 to their overall health state with a higher number indicating a better overall health state. The median overall distribution for the EQ-5D index and the VAS score was used as a cut-off to define low HRQoL. This analysis included 2,205 patients at baseline, 1,861 at the 6-month visit, and 1,506 at the 12-month visit. The median age at baseline was 74 years (IQR, 67– 80).
Key findings
- The median EQ-5D index was 0.779 and the median VAS score was 0.700.
- In the univariable analyses of EQ-5D and VAS, age, sex, Revised International Prognostic Scoring System (IPSS-R), serum ferritin levels, Hb and neutrophil counts, receiving erythropoiesis-stimulating agents, red blood cell (RBC) transfusions or iron chelators, Sorror and MDS-specific comorbidity indexes, KPS, and body mass index were included as potential determinants of low HRQoL. The lowest category of platelet count was associated with low VAS.
- The multivariable analysis showed that age >75 years (p < 0.001), sex (female, p < 0.001), serum ferritin levels ≥1000 μg/L (p = 0.018), high MDS comorbidity index (p = 0.011), and lower KPS (p < 0.001) were determinants of low EQ-5D.
- The multivariable analysis also showed that age >75 years (p = 0.015), sex (female, p = 0.032), serum ferritin levels ≥1000 μg/L (p = 0.034), high MDS comorbidity index (p = 0.002), lower KPS (p < 0.001), transfusion dependence (p = 0.037), Hb value <10 g/dL (p = 0.001), and a high body mass index >30 kg/m2 (p = 0.033) were associated with low VAS.
- Within the five EQ-5D dimensions, univariable analyses revealed the following:
- Sex (female), reduced KPS, high Sorror comorbidity index, low Hb count, and transfusion-related variables are potential determinants of low HRQoL in all dimensions.
- Increased age, serum ferritin levels, neutrophil count, and MDS comorbidity index were relevant in all other dimensions but not in the anxiety/depression dimension.
- IPSS-R and sex were not relevant for the pain and discomfort dimension.
- Increased age was particularly relevant for the mobility and self-care dimensions.
Red blood cells transfusion burden in low-risk MDS3
Study design and patient characteristics
This comparative observational study used patient data from the Canadian MDS (MDS-CAN) registry. The effect of RBC transfusions on HRQoL was assessed by calculating the RBC dose densities as the cumulative RBC transfusions divided by the number of days until the completion of the EQ-5D. In total, 145 patients with MDS with ring sideroblasts who were IPSS-R very low-, low-, or intermediate-risk were included in the MDS-CAN registry, with a mean age of 73.0 years.
Key findings
- 127 patients completed ≥1 EQ-5D-3L questionnaire, with a median of 4 (IQR: 2–8) and the mean baseline index was 0.786.
- Dose density of RBC transfusions was associated with a reduction in EQ-5d-3L index scores
- before adjusting for baseline and time-varying covariates (mean difference, −0.0068; 95% credible interval, −0.0092 to −0.0043); and
- after adjusting for baseline and time-varying covariates (mean difference, −0.0063; 95% credible interval, −0.0091 to −0.0034).
- Estimates for the rate of RBC transfusions did not reach statistical significance after adjusting for baseline and time-varying covariates.
Conclusion
The patient’s voice is recognized as valuable information in both clinical research and practice; however, it can be difficult to quantify this data. Efficace et al.1 provided a successful validation of the QUALMS and the three subscales were established. This scale could help to collect HRQoL data with a specific focus on patients with MDS. Several factors such as sex, KPS, comorbidity burden, Hb count, and transfusion burden influenced HRQoL in a large international population of real-world patients with MDS. Furthermore, there was an association between treatment-related burdens from RBC transfusion factors and HRQoL in patients with lower-risk MDS with ring sideroblasts. Overall, identifying and considering factors that influence HRQoL may improve the quality of care and aid in patient-shared decision making.2 Additionally, incorporating patient-reported outcomes in practice can be helpful in patient counseling, personalized clinical decision-making, and in clinical trial design to identify patients for whom intervention is necessary.2
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

