All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Updates from NCRI AML18 and AML19 trials
During the 2nd National Cancer Research Institute (NCRI) acute myeloid leukemia (AML) Academy Meeting, Nigel Russell presented updated data on the ongoing NCRI AML18 and AML19 trials.1 Here, we are pleased to summarize the outcomes from these trials.
The AML18 trial (NCT02272478) is an ongoing phase II/III trial in older patients with AML and high-risk myelodysplastic syndrome (MDS). This is a comparative trial of daunorubicin/Ara-C (DA) and gemtuzumab ozogamicin (GO) against CPX-351, in older patients (> 60 years) with regards to overall survival (OS) and safety. It also further investigates the clinical value of measurable residual disease (MRD) following the first treatment course.1
AML18 trial
The original design compared DA plus a single dose of GO (DAGO1, 3 mg/m2) versus DA plus two fractionated doses of GO (DAGO2, maximum 5 mg/m2) in patients with no known adverse cytogenetics, who were fit for intensive chemotherapy. This comparison evaluated if intensified treatment would improve outcomes in patients with MRD positivity following the first treatment course.1
Nigel Russell presented data from this original study design during his talk in comparison to results from another trial, the phase II/III AML16 trial (NCT00454480), which investigated different chemotherapy combinations with or without GO or tipifarnib in patients with AML or high-risk MDS.
Methods and patients
A total of 847 patients were randomized (see Figure 1). Following the first treatment course with either DAGO1 or DAGO2, patients were re-randomized to intensified treatment arms to demonstrate if patients who were not in complete remission (CR), or those who were in CR with MRD positivity, would benefit from this approach. However, the 2nd randomization was not a subject for this talk.
Figure 1. Randomization1
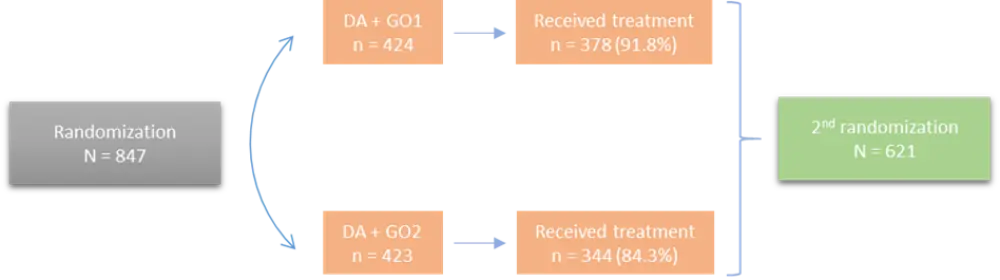
DA, daunorubicin/Ara-C; GO, gemtuzumab ozogamicin.
The study population consisted of older patients with AML or high-risk MDS, with a median age of 68 years (range, 56–81). Other characteristics are summarized in Table 1.
Table 1. Baseline characteristics1
|
AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; WBC, white blood cells; WHO PS, World Health Organization performance status |
|
|
Characteristic |
% of patients |
|
Sex Male Female |
60.5 39.5 |
|
Diagnosis De novo AML Secondary AML MDS |
79.6 10.4 10 |
|
WHO PS 0 1 2 |
48 45 7 |
|
Median WBC, × 109/L (range) |
4.7 (0.2–336.7) |
|
Cytogenetics Favorable Intermediate Adverse Unknown |
4 76 14 6 |
Outcomes
There was no significant difference in supportive care (such as hospitalization) or hematological recovery between both groups, although there was a slight increase in platelet count in the DAGO2 arm (p = 0.04). Safety analysis showed that the DAGO2 regimen was not associated with greater liver toxicity compared with DAGO1, suggesting that the fractionated dose was well tolerated.
Response and survival outcomes among study arms are presented in Table 2. Briefly, there were no significant differences in OS, Day 30+ mortality or 3-year cumulative incidence of relapse between the DAGO1 and DAGO2 arms. With regards to response rates, there was only a marginally higher complete remission with incomplete hematological recovery (CRi) rate in DAGO2-receiving patients following the first treatment course (Table 2).
Table 2. Efficacy outcomes1
|
CI, confidence interval; CR, complete remission; CRi, CR with incomplete hematological recovery; DA, daunorubicin/Ara-C; GO, gemtuzumab ozogamicin; MRD, measurable residual disease; OR, odds ratio; OS, overall survival, SCT, stem cell transplant. Significant differences between groups are shown in bold. *From time of remission (CR/CRi) |
||||
|
Outcome, % |
DAGO1 |
DAGO2 |
OR (95% CI) |
p value |
|
After Course 1 |
||||
|
CR |
62.7 |
64.9 |
1.10 (0.83–1.46) |
0.5 |
|
CRi |
5.5 |
9.0 |
1.70 (0.99–2.92) |
0.05 |
|
CR + CRi |
68.2 |
74.0 |
1.32 (0.98–1.79) |
0.06 |
|
CR/CRi MRD negative |
54.0 |
57.2 |
1.14 (0.82–1.59) |
0.44 |
|
After Course 1 + 2 |
||||
|
CR + CRi |
77.4 |
78.4 |
1.06 (0.76–1.48) |
0.72 |
|
Day 30+ mortality |
7.3 |
7.8 |
1.07 (0.64–1.79) |
0.79 |
|
OS |
||||
|
3-year OS |
36.0 |
36.5 |
0.94 (0.76–1.11) |
0.375 |
|
OS from remission |
42.0 |
41.1 |
― |
― |
|
3-year OS excluding adverse-risk patients |
40.0 |
42.76 |
0.90 (0.73–1.10) |
0.3 |
|
3-year cumulative incidence of relapse* |
50.2 |
49.6 |
0.96 (0.79–1.17) |
0.71 |
|
Patients who underwent SCT in CR1 |
38.0 |
30.0 |
― |
― |
Comparison of outcomes between the AML16 and AML18 trials is shown in Figure 2. However, Nigel Russel emphasized that these trials are not strictly comparable.
Figure 2. Comparative data from AML16 and AML18 trials1
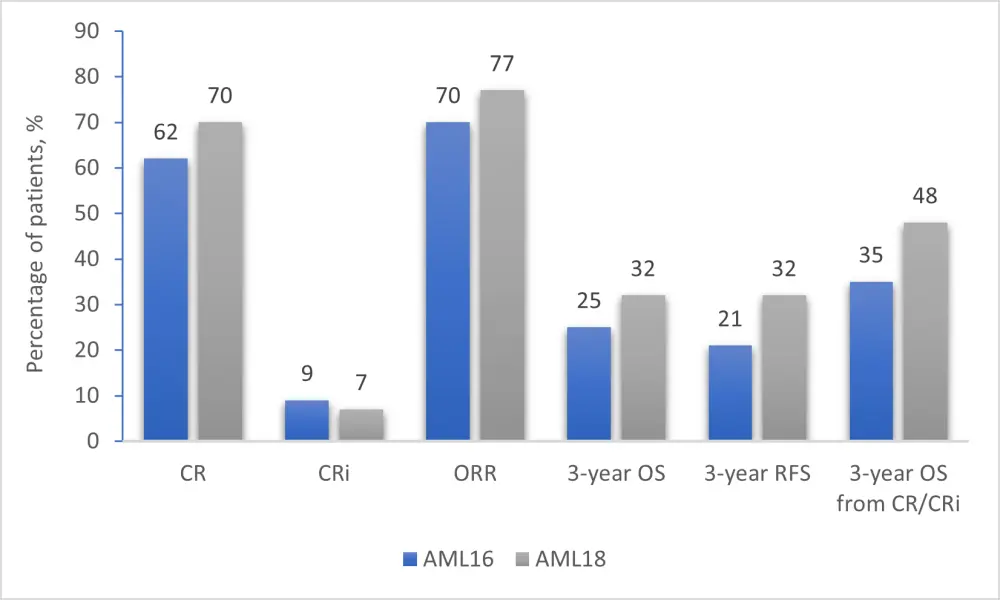
AML, acute myeloid leukemia; CR, complete remission; CRi, CR with incomplete hematological recovery; ORR, overall response rate; OS, overall survival, RFS, relapse-free survival.
Three-year OS by MRD status following Course 1 in patients with CR/CRi was 51.1% (vs 24% in the AML16 trial) for MRD-positive patients, and 46.4% (vs 46% in the AML16 trial) for MRD-negative patients (p = 0.84). MRD status could not identify patients who would benefit more from allogeneic stem cell translation in CR1, as all analyzed groups (MRD negative, MRD positive, MRD unknown, no CR post Course 1) showed no differential effects.
In patients with NPM1 mutation (n = 212) the CR rate after Course 1 was 79%, MRD negativity rate was 66%, and 3-year OS was 50.6% (3-year OS was 25% in the AML16 trial).
Conclusion
This trial demonstrated no evidence of improvement in survival with the fractionated GO regimen (DAGO2) compared with the single dose (DAGO1). However, there was a trend for higher response rates after induction Course 1 with DAGO2. The toxicity profile was similar for both regimens, except a slightly higher platelet count in the DAGO2 arm. Patients with MRD-positive status and those with MRD-negative status had similar outcomes. Patients with NPM1 mutations had superior outcomes compared with NPM1 wild type.
AML19 trial
Nigel Russell also gave a brief update on the AML19 trial, which started in November 2015, and recruited 1891 patients until a temporary suspension in recruitment in December 2019. The trial will reopen in mid-October 2020 and aims to enroll approximately 250 patients over a 1-year period who will be randomized to either DAGO1 or DAGO2, and patients with FLT3 mutation (n = ~50) will receive midostaurin. The trial will continue to compare DAGO1 and DAGO2 regimens in patients with de novo AML and will investigate the safety and efficacy of midostaurin in FLT3-mutated patients who received DAGO treatment. Eligibility criteria include:
- De novo CD33+ AML with favorable, intermediate-risk or unknown cytogenetics based on the World Health Organization (WHO) classification
- WHO performance status 0–2
- Suitable for intensive chemotherapy
- Aged 16–60:
- Patients aged > 60 are deemed eligible if they are suitable for intensive chemotherapy
- For the midostaurin arm, patients must be ≥ 18 years of age and have FLT3 tyrosine kinase domain (TKD) or internal tandem duplication (ITD) mutation
- Serum alanine aminotransferase and aspartate aminotransferase ≤ 2.5 × ULN, and bilirubin ≤ 2 × ULN
Patients will be randomized to either DAGO1 or DAGO2 for Course 1. Then they will be assessed for FLT3/NPM1 status, and patients with FLT3 mutation will receive midostaurin. Following risk assessment, high-risk patients will continue with standard of care treatment and standard-risk patients will receive DA monotherapy in Course 2. MRD status will be assessed at the end of Course 2, and standard risk patients will continue on high-dose cytarabine, and high-risk patients will receive standard of care treatment.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

