All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
The impact of post-transplant MRD on survival in AML and MDS: An analysis from the phase II FIGARO trial
Do you know... In patients with MDS who receive a reduced-intensity conditioning regimen and allogeneic hematopoietic stem cell transplantation, which of the following statements is true?
Reduced-intensity conditioning (RIC) regimens allow patients with acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) who are unfit for myeloablative conditioning regimens to receive allogeneic hematopoietic stem cell transplantation (allo-HSCT). However, the prognostic impact of post-transplant T-cell chimerism and measurable residual disease (MRD) in patients with AML or MDS receiving RIC allo-HSCT has not been assessed prospectively.1
The AML Hub previously reported results from the randomized phase II FIGARO trial of patients receiving allo-HSCT for AML or MDS, comparing a fludarabine + cytarabine + amsacrine + busulfan + anti-thymocyte globulin (FLAMSA-Bu) RIC regimen with the investigator’s standard fludarabine-based RIC regimen choice.1 The AML Hub has also previously published a video interview with Charles Craddock discussing predictors of outcomes post-transplant from this trial. Recently, Loke et al.1 published further analyses from the trial in Blood Advances assessing the impact of post-transplant MRD and T-cell chimerism status on survival outcomes.
Study design and patient characteristics
The study design of the FIGARO trial has been covered previously. Serial samples for MRD analysis, assessed by flow cytometry, were collected pre-transplant, and for 187 patients, post-transplant at Day 42, and Months 3, 6, 9, and 12. Peripheral blood samples for T-cell chimerism were collected every 3 months during the first year post-transplant. The median follow-up was 49.7 months.
Key findings
Post-transplant MRD frequency
- In the first 12 months after allo-HSCT, 16% of patients had ≥1 MRD-positive (MRD+) sample.
- MRD positivity was most frequently noted on Day 42 (n = 13) and Month 3 (n = 12), then decreasing over time.
- Baseline disease and transplant characteristics were similar between MRD+ patients and MRD negative (MRD−) patients (Table 1).
- Multivariate analysis showed that MRD pre-transplant was the only factor associated with post-transplant MRD (p = 0.03).
Table 1. Baseline characteristics*
|
AML, acute myeloid leukemia; ATG, anti-thymocyte globulin; Bu, busulfan; FLAMSA, fludarabine + cytarabine + amsacrine; Flu, fludarabine; IPSS, International Prognostic Scoring System; MDS, myelodysplastic syndromes; MRD, measurable residual disease; RIC, reduced-intensity conditioning. |
||||
|
Characteristics, % unless otherwise specified |
Overall |
Post-transplant MRD status |
p value |
|
|---|---|---|---|---|
|
Positive at any time point (n = 29) |
Negative only (n = 158) |
|||
|
RIC regimen |
|
|
|
|
|
FLAMSA-Bu |
50 |
34 |
49 |
0.21 |
|
Flu/Bu/ATG |
29 |
31 |
32 |
- |
|
Flu/melphalan/alemtuzumab |
14 |
21 |
13 |
- |
|
Flu/Bu/alemtuzumab |
7 |
14 |
6 |
- |
|
Age |
|
|
|
|
|
≤60 years |
58 |
66 |
59 |
0.61 |
|
>60 years |
42 |
34 |
41 |
- |
|
Sex |
|
|
|
|
|
Female |
42 |
38 |
43 |
0.97 |
|
Male |
58 |
62 |
57 |
- |
|
Underlying disease |
|
|
|
|
|
AML |
67 |
69 |
64 |
0.44 |
|
MDS |
33 |
31 |
36 |
- |
|
Patients with cytogenetic risk-AML |
|
|
|
|
|
Adverse risk |
31 |
45 |
23 |
0.11 |
|
Intermediate risk |
64 |
45 |
71 |
- |
|
Favorable risk |
5 |
10 |
5 |
- |
|
Unknown |
1 |
- |
1 |
- |
|
FLT3 |
|
|
|
|
|
Absent |
40 |
38 |
39 |
0.76 |
|
Present |
17 |
24 |
18 |
- |
|
NPM1 |
|
|
|
|
|
Absent |
41 |
52 |
39 |
0.90 |
|
Present |
16 |
10 |
18 |
- |
|
IPSS (MDS only) |
|
|
|
|
|
Standard risk (≤2) |
97 |
100 |
96 |
1 |
|
High risk (>2) |
3 |
- |
4 |
- |
|
Donor type |
|
|
|
|
|
Sibling |
21 |
28 |
20 |
0.82 |
|
Unrelated |
79 |
72 |
80 |
- |
|
Pre-transplant MRD |
|
|
|
|
|
Positive |
20 |
34 |
20 |
0.03 |
|
Negative |
52 |
41 |
56 |
- |
|
Missing |
28 |
24 |
24 |
- |
Impact of MRD on survival outcomes
- Post-transplant MRD positivity was associated with inferior overall survival (OS) and relapse-free survival (RFS) (Table 2).
- Cox model analysis with post-transplant MRD status as a time-dependent variable showed that MRD+ patients had inferior OS (hazard ratio [HR], 2.18; 95% confidence interval [CI], 1.31–3.62; p = 0.0028) and RFS (HR, 5.32; 95% CI, 3.27–8.68; p < 0.001).
- Multivariate analysis accounting for other prognostic factors, including FLT3 status, cytogenetic risk, and chronic graft-versus-host disease (GvHD), demonstrated that post-transplant MRD remained predictive of outcomes, independent of pre-transplant MRD status.
- Post-transplant MRD was associated with inferior OS in patients who were MRD+ pre-transplant (adjusted HR, 2.70; 95% CI, 1.76–4.15; p < 0.001) and MRD− pre-transplant (adjusted HR, 2.68; 95% CI, 1.79–4.03; p < 0.001).
- Adverse cytogenetics was also associated with inferior OS in patients who were MRD− pre-transplant (adjusted HR, 1.61; 95% CI, 1.08–2.39; p = 0.019).
Table 2. 2-year estimated survival outcomes based on MRD status at each time point*
|
CI, confidence interval; CIR, cumulative incidence of relapse; MRD, measurable residual disease; OS, overall survival; RFS, relapse-free survival. |
||||
|
Outcome |
Time point |
Post-transplant MRD status |
||
|---|---|---|---|---|
|
MRD+ 2-year estimate (95% CI) |
MRD− 2-year estimate (95% CI) |
p value |
||
|
CIR |
Day 42 |
92.3 (35.8–99.4) |
22.9 (16.4–30.1) |
<0.001 |
|
Month 3 |
50.0 (19.2–74.8) |
20.5 (14.0–27.7) |
0.011 |
|
|
Month 6 |
83.3 (8.6–98.7) |
17.4 (11.1–24.9) |
<0.001 |
|
|
Month 9 |
100 |
14.6 (8.5–22.1) |
<0.001 |
|
|
Month 12 |
50.0 (0.0–96.0) |
14.0 (8.0–21.6) |
0.19 |
|
|
OS |
Day 42 |
30.8 (9.5–55.4) |
66.9 (58.5–74.0) |
<0.001 |
|
Month 3 |
58.3 (27.0–80.1) |
74.0 (65.5–80.6) |
0.30 |
|
|
Month 6 |
50.0 (11.1–80.4) |
80.6 (72.0–86.8) |
<0.0001 |
|
|
Month 9 |
33.3 (0.9–77.4) |
87.8 (79.5–92.9) |
<0.0001 |
|
|
Month 12 |
50.0 (0.6–91.0) |
94.7 (87.8–97.8) |
0.18 |
|
|
RFS |
Day 42 |
7.7 (0.5–29.2) |
61.0 (52.6–68.5) |
<0.001 |
|
Month 3 |
50.0 (20.8–73.6) |
67.3 (58.6–74.6) |
0.13 |
|
|
Month 6 |
16.7 (0.8–51.7) |
75.4 (66.3–82.3) |
<0.001 |
|
|
Month 9 |
33.3 (0.9–77.4) |
79.3 (69.7–86.2) |
<0.001 |
|
|
Month 12 |
50.0 (0.6–91.0) |
83.7 (74.4–89.9) |
0.31 |
|
Impact of mixed donor T-cell chimerism on survival outcomes
- Of the 155 patients with sequential chimerism status data available, 52 had mixed donor T-cell chimerism at Month 3 while being relapse-free.
- Patients with mixed T-cell chimerism had inferior OS and RFS.
- Multivariate Cox model analysis of OS with chimerism as a time-dependent variable and excluding MRD revealed that acquiring full donor T-cell chimerism improved OS (HR, 0.33; 95% CI, 0.17–0.66; p = 0.0018).
Interaction between T-cell chimerism and post-transplant MRD
T-cell chimerism and post-transplant MRD data up to Month 6 were available for 94 patients.
- Of patients with full donor T-cell chimerism (n = 47) at both Months 3 and 6, 10.6% were MRD+, whereas of those with mixed donor T-cell chimerism (n = 47) at Months 3 and 6, 29.8% were MRD+ before or at the time of mixed donor chimerism (p = 0.018).
- The presence of MRD impacted 2-year OS and RFS in patients with mixed donor-T-cell chimerism but not in those with full donor chimerism (Figure 1).
- A similar pattern was observed up to 1 year post-transplant:
- MRD positivity was observed only in 7% of patients with full donor T-cell chimerism for ≥2 sequential time points.
- Of the 24 patients who were MRD− up to observed mixed donor T-cell chimerism, 21% converted to MRD+ or relapsed within 3 months, with only 1 patient relapsing in the subsequent 2 years.
Figure 1. Impact of T-cell chimerism and post-transplant MRD status on estimated 2-year survival outcomes*
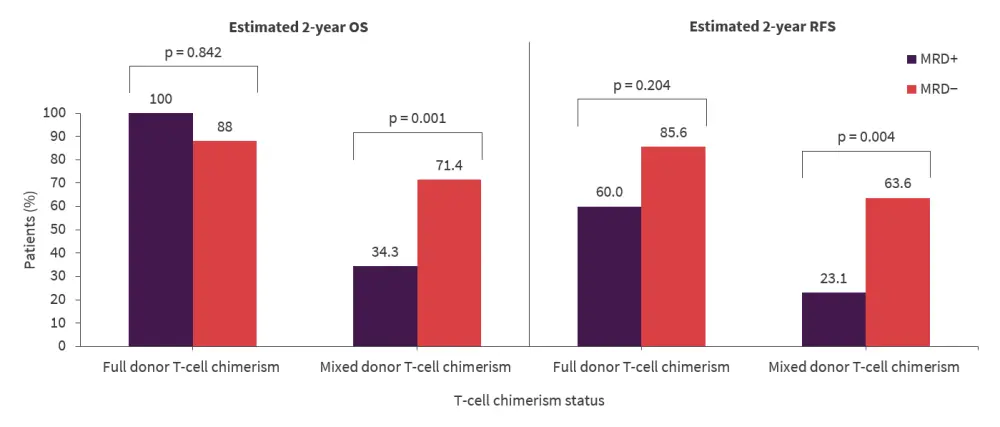
MRD, measurable residual disease; OS, overall survival; RFS, relapse-free survival.
*Data from Loke et al.1
HLA-DR downregulation and post-transplant MRD
It is thought that the graft-versus-leukemia effect of allo-HSCT may be affected by downregulation of HLA Class II molecules on the surfaces of leukemic blasts; therefore, the association of increased risk of relapse with MRD positivity and HLA-DR downregulation was investigated.
- 34% of patients with post-transplant MRD positivity had HLA-DR-negative blasts (HLA-DR downregulation), all of whom relapsed.
- These patients also had worse 2-year OS (20.0% vs 57.9%; p < 0.001) and RFS (10.0% vs 47.4%; p < 0.001) than those who did not have the presence of HLA-DR-negative-blasts.
Conclusion
This analysis from the FIGARO trial demonstrated the prognostic significance of post-transplant MRD, independent of pre-transplant MRD status, in patients with AML or MDS who underwent allo-HSCT using a RIC regimen. This analysis also showed the impact of T-cell chimerism on transplant outcomes and the interaction with post-transplant MRD, and that HLA-DR blast expression can provide further prognostic information in patients who are MRD+ post-transplant.
The authors concluded that the results from this study support combined MRD and T-cell donor chimerism monitoring for patients with AML or MDS following RIC allo-HSCT.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

