All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Sabatolimab for the treatment of poor prognosis myelodysplastic syndromes and acute myeloid leukemia
High-risk and very high-risk myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) carry poor prognosis for patients despite advances in treatment options. Sabatolimab is a novel antibody that targets T-cell immunoglobulin and mucin domain-containing protein 3 (TIM-3), a putative checkpoint receptor involved in regulating both innate and adaptive immune responses. TIM-3 is expressed on leukemic stem cells, but not normal hematopoietic stem cells. Sabatolimab binds directly to leukemic cells, driving the immune-mediated killing of tumor cells, and blocking replication.1
At the 26th Congress of the European Hematology Association (EHA2021), Andrew Wei and colleagues1 presented findings from a phase Ib clinical trial (NCT03066648), which characterized the safety and tolerability of sabatolimab with hypomethylating agents (HMAs) in patients with poor prognosis MDS and AML. The findings are summarized below.
Study design
A phase Ib clinical trial, with the study design shown in Figure 1.
Figure 1. Study design*
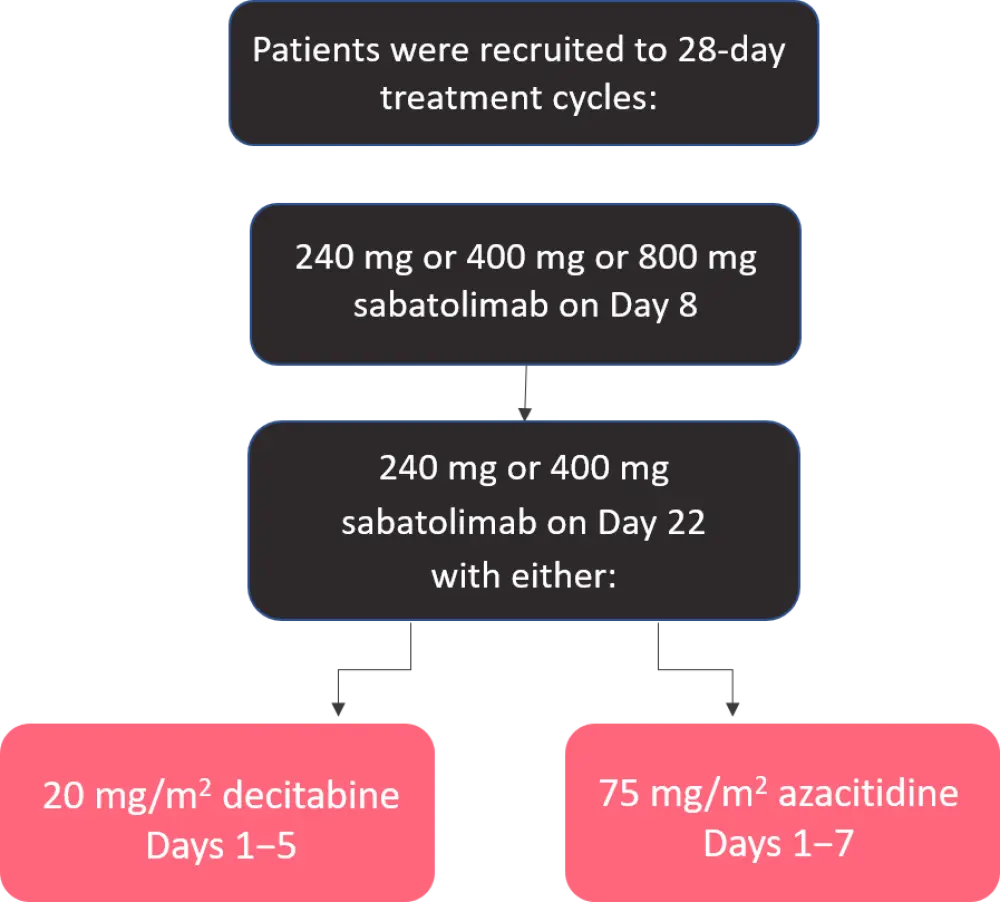
*Adapted from Wei et al.1
Inclusion criteria
Revised International Prognostic Scoring System (IPSS-R) high-risk or very high-risk MDS or newly diagnosed unfit AML patients, ineligible for standard chemotherapy.
Endpoints were as follows:
- Primary endpoints: maximum tolerated dose; recommended dose; safety and tolerability.
- Secondary endpoints: preliminary efficacy; response rates; duration of response.
Results
A total of 101 patients were recruited. Table 1 shows basic demographic and diagnostic data.
Table 1. Demographic and diagnostic data*
|
AML, acute myeloid leukemia; HR, high-risk; MDS, myelodysplastic syndromes; vHR, very high-risk. |
||
|
Characteristic |
HR/vHR MDS |
AML |
|---|---|---|
|
Sabatolimab + decitabine patients, n |
19 |
22 |
|
Sabatolimab + azacitidine patients, n |
34 |
26 |
|
Median age, years |
70 (range, 23−90) |
75 (range, 59−89) |
|
Male, n (%) |
29 (54.7) |
26 (54.2) |
|
Risk category, n (%) |
||
|
High |
32 (60.4) |
— |
|
Very high |
21 (39.6) |
— |
|
Intermediate |
— |
19 (39.6) |
|
Adverse |
— |
29 (60.4) |
Secondary endpoints: Safety and tolerability
Of the 101 patients, one patient in the AML group discontinued treatment due to a sabatolimab-related adverse event, and there was one sabatolimab-related death in the MDS group (neutropenic colitis with shock). Only one patient discontinued treatment due to sabatolimab dose-limiting toxicity (hepatitis) (Table 2). Overall, treatments were well tolerated with median treatments in the MDS and AML groups of 8.01 and 6.8 months, respectively.
Table 2. Tolerability and continuation data for treatment groups by condition*
|
AE, adverse event; AML, acute myeloid leukemia; HR, high-risk; MDS, myelodysplastic syndromes; SCT, stem cell transplantation; vHR, very high-risk. |
||
|
Tolerability |
HR/vHR MDS |
AML |
|---|---|---|
|
Sabatolimab + decitabine, median treatment (months) |
8.02 |
6.8 |
|
Sabatolimab + azacitidine, median treatment (months) |
3.84 |
5.98 |
|
Patients discontinuing therapy, n (%) |
39 (74) |
42 (87.5) |
|
Reasons for discontinuation, n (%) |
||
|
SCT |
12 (23) |
0 |
|
AE related to sabatolimab |
0 |
1 (2) |
|
Death, related to sabatolimab |
1 (2) |
0 |
|
Dose-limiting toxicity |
0 |
1 (3) |
Most of the adverse events were attributable to HMAs alone, not sabatolimab, with 35% of patients requiring a dose interruption, and 1% requiring a dose reduction. The most common events were either hematopoietic-related (thrombocytopenia, neutropenia, anemia) or gastrointestinal (nausea, constipation, diarrhea).
In terms of immune-mediated adverse events, only four patients experienced possible treatment-related (sabatolimab) events, leading to dose delay, reduction, or discontinuation.
In terms of clinical outcomes, the overall risk rate was 58% for MDS patients, and 42.5% for AML patients. Further data relating to efficacy and duration of response can be seen in Table 3, including data on duration of response in patients with adverse risk disease (e.g., TP53 mutations, cytogenetic poor risk, and at least one of the TP53/RUNX1/ASXL1 mutations).
Table 3. Clinical outcomes and survival data for both MDS and AML patients*
|
AML, acute myeloid leukemia; CR, complete response; DOR, duration of response; HR, high-risk; MDS, myelodysplastic syndromes; NE, not evaluable; ORR, overall response rate; PFS, progression-free survival; vHR, very high-risk. |
||||
|
Outcome |
HR/vHR MDS |
AML |
||
|---|---|---|---|---|
|
Overall DOR, months (95% CI) |
16.1 |
12.6 |
||
|
Estimated 12-month PFS, % (95% CI) |
50.1 |
27.4 |
||
|
Patients in CR in the intention-to-treat population, % |
20 |
25 |
||
|
DOR in CR, months (95% CI) |
21.5 |
23.0 |
||
|
Mutation |
ORR, % |
DOR, months |
ORR, % |
DOR, months |
|
TP53 |
71.4 |
14.7 |
40 |
4.2 |
|
Cytogenetic poor risk |
60 |
12.1 |
— |
— |
|
At least one of the following: TP53/RUNX1/ASXL1 |
67.7 |
16.1 months |
53.8 |
12.6 months |
Conclusion
Overall, the data from this phase Ib trial of sabatolimab with HMAs demonstrate that the combination is well tolerated in patients with poor prognosis MDS and AML. It also demonstrates that complete remission, and durable disease responses can be achieved in both patient groups, including those with adverse risk disease, such as TP53 mutations. Further phase II and phase III clinical trials are needed, and currently underway, to determine what benefit patients with poor prognosis MDS and AML could derive from this treatment combinations.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

