All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Revised International Working Group 2023 response criteria for higher-risk MDS
The International Working Group (IWG) response criteria for myelodysplastic syndromes (MDS) were developed in 2000 and revised in 2006 and 2018.1 Previous editions of the IWG response criteria have limited applicability in higher-risk MDS (HR-MDS), with the inability to fully capture the benefits of novel agents or assist as valid surrogate end-points for longer-term clinical outcomes.1
To address these issues and to improve the clarity and practicality of the response criteria, an international panel of 36 MDS experts employed a modified Delphi process to develop the consensus IWG 2023 response criteria.1 These revised IWG response criteria for HR-MDS were published by Zeidan et al.1 in Blood, with the aim of updating the criteria for better correlation between patient-centered outcomes and clinical trial results. Below, we summarize the key changes.
Key updates1
Response definition
- Response assessment per the IWG 2023 MDS response criteria is detailed in Figure 1.
- Response assessment for patients with <5% bone marrow (BM) at baseline is detailed in Figure 2.
Figure 1. Response assessment flow chart per IWG 2023 MDS response criteria*
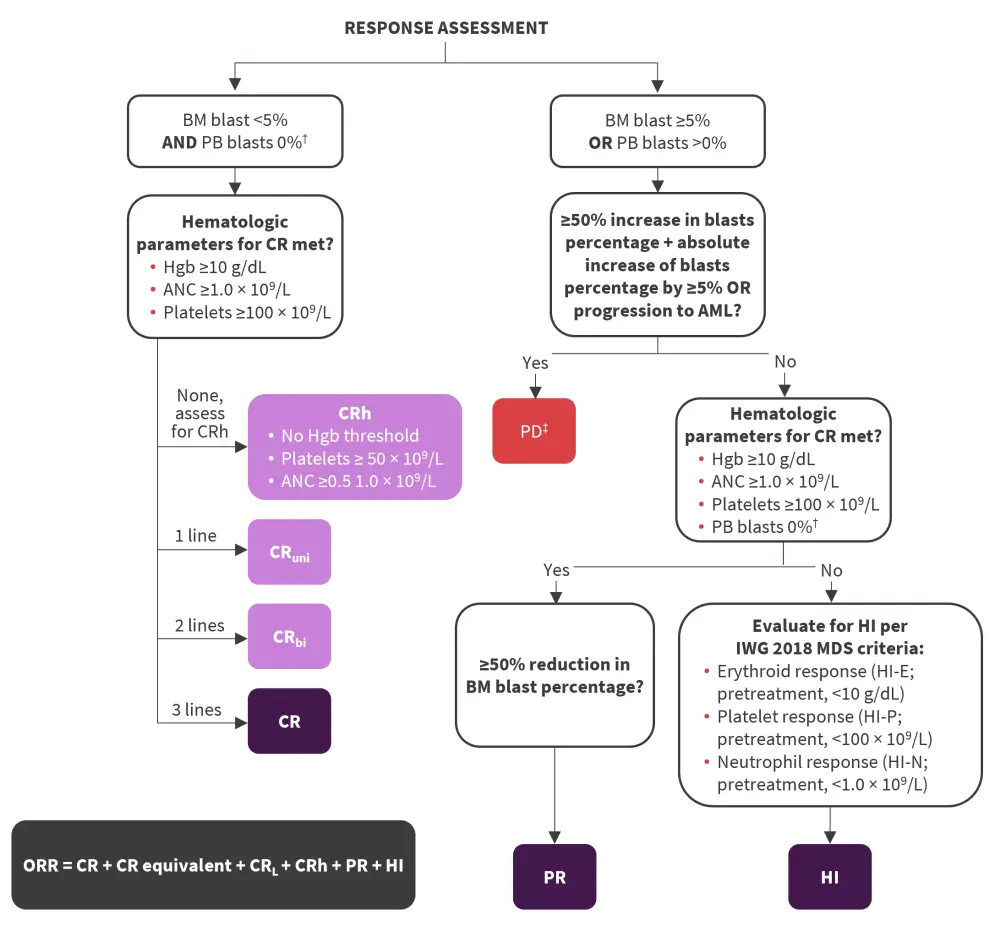
AML, acute myeloid leukemia; ANC, absolute neutrophil count; BM, bone marrow; CR, complete remission; CRbi, CR with bilineage hematologic improvement; CRh, CR with partial hematologic recovery; CRL, CR with limited count recovery; CRuni, CR with unilineage hematologic improvement; Hgb, hemoglobin; HI, hematologic improvement; HI-E, HI erythroid response; HI-N, HI neutrophil response; HI-P, HI platelet response; IWG, International Working Group; MDS, myelodysplastic syndromes; ORR, overall response rate; PB, peripheral blood; PD, progressive disease; PR, partial remission.
*Adapted from Zeidan, et al.1
†Response assessment allows for 2 weeks before or after date of BM assessment to allow regeneration of blood counts and clearance of PB blasts without the need for a repeat BM biopsy.
‡New red blood cell or platelet transfusion requirements also constitutes PD.
Figure 2. Response assessment flowchart for patients with <5% BM at baseline*
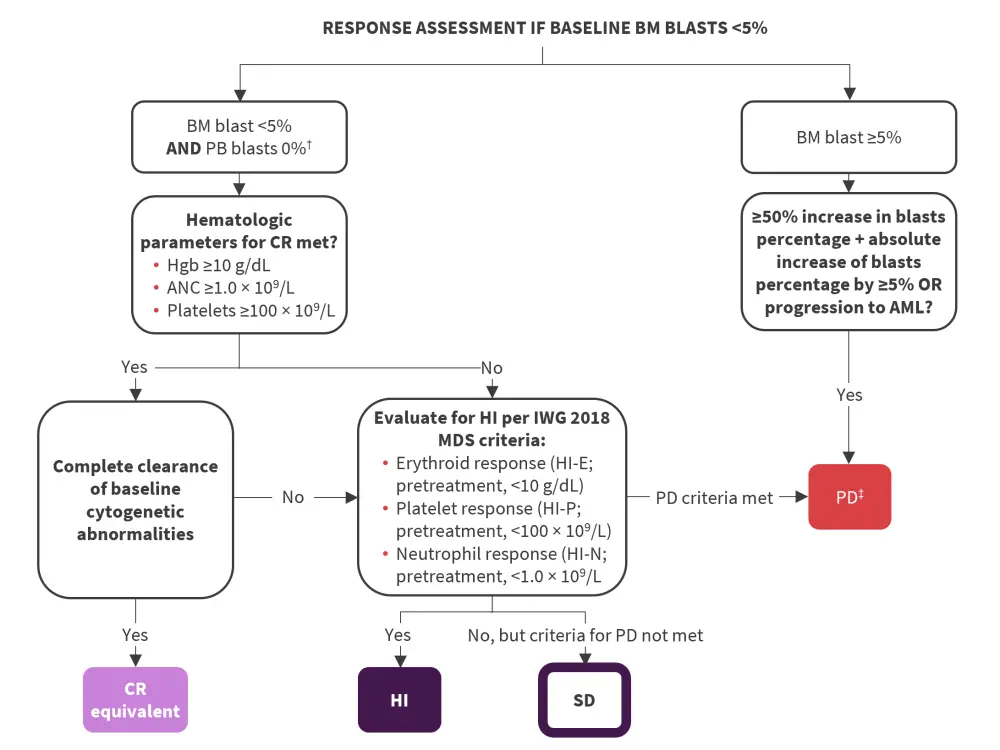
AML, acute myeloid leukemia; ANC, absolute neutrophil count; BM, bone marrow; CR, complete remission; Hgb, hemoglobin; HI, hematologic improvement; HI-E, HI erythroid response; HI-N, HI neutrophil response; HI-P, HI platelet response; IWG, International Working Group; MDS, myelodysplastic syndromes; PB, peripheral blood; PD, progressive disease; PR, partial remission; SD, stable disease.
*Adapted from Zeidan, et al.1
†Response assessment allows for 2 weeks before or after date of BM assessment to allow regeneration of blood counts and clearance of PB blasts without the need for a repeat BM biopsy.
‡New red blood cell or platelet transfusion requirements also constitutes PD.
Complete remission
- A BM blasts threshold of <5% is proposed to define complete remission (CR) in MDS in line with the Revised International Prognostic Scoring System (IPSS-R), 2022 MDS World Health Organization (WHO) classification, and European LeukemiaNet (ELN) acute myeloid leukemia (AML) response criteria.
- The proposed hemoglobin (Hgb) threshold for CR is lowered from <10 g/dL to ≥10 g/dL to reflect clinically meaningful erythroid recovery unlikely to be associated with ongoing red blood cell transfusion requirements.
- The thresholds for platelets (≥100 × 109/L) and absolute neutrophil count (ANC; ≥1.0 × 109/L) remain unchanged.
- While only patients with ≥5% BM blasts prior to treatment are eligible for CR assessment, in patients with HR-MDS with <5% blasts due to cytogenetics and/or severe cytopenias, a complete cytogenetic response and Hgb ≥10 g/dL, platelets ≥100 × 109/L, and ANC ≥1.0 × 109/L can be regarded as a CR equivalent.
Less than CR response
- CR with limited count recovery (CRL) is proposed to replace marrow CR.
- CRL has the same thresholds as CR and can occur in one lineage as CR unilineage or in two lineages as CR bilineage.
- CR with partial hematologic recovery (CRh), defined as BM blasts <5%, ANC ≥0.5 × 109/L, and platelet count ≥50 × 109/L, is included for patients with MDS/AML or MDS with increased blasts (MDS-IB2), defined by the International Consensus Classification (ICC) and WHO 2022 classifications, respectively.
- Baseline peripheral blood (PB) counts are not considered when defining CRL and CRh.
Partial remission and stable disease
- Partial remission is defined as BM blasts reduction by ≥50% to ≥5% with Hgb ≥10 g/dL, platelets ≥100 × 109/L, and ANC ≥1.0 × 109/L.
- Stable disease should not be included as part of the overall response rate as it may not necessarily mean treatment failure in patients treated with hypomethylating agents.
Overall response rate
- Overall response rate is defined as a composite of CR, CR equivalent, partial remission, CRL, CRh, and hematologic improvement.
- Marrow CR and stable disease should not be included if reported due to a lack of association with improved overall survival (OS).
Cytogenetic response and measurable residual disease assessment
- Complete and partial cytogenetic responses as defined by the 2006 IWG response criteria should continue to be reported.
- Measurable residual disease can be reported as a provisional response category in clinical trials.
- Molecular endpoints should be reported whenever possible.
Not evaluable as a response category for clinical trials
- Not evaluable encompasses patients yet to have a response assessment, suffering premature death, leaving the study early, or with unsatisfactory BM samples.
Progressive disease
- Progressive disease (PD) can be defined as progression based on the increasing of BM or PB blasts (PD-B), worsening cytopenias/transfusion requirements (PD-C), or progression to AML (PD-AML).
Disease relapse
- Disease relapse is defined by fulfilling one of the following criteria:
- BM blasts increase by ≥5% and ≥50% increase from prior assessment.
- The confirmed reappearance of blasts in the blood.
- Reduction in PB counts defined as either a ≥50% decline from maximum remission/response levels in granulocytes or platelets, a reduction in Hgb by 1.5 g/dL, or transfusion dependence or development of extramedullary disease.
Time-to-event based outcomes
- OS should continue to be the primary endpoint for phase III clinical trials.
- Event-free survival and progression-free survival can serve as a proxy for OS; although further prospective validation is needed.
- The proposed standardized definitions for clinical trials are detailed in Figure 3.
Figure 3. Updates to the IWG time-to-event endpoints for higher-risk MDS enrolled in clinical trials*
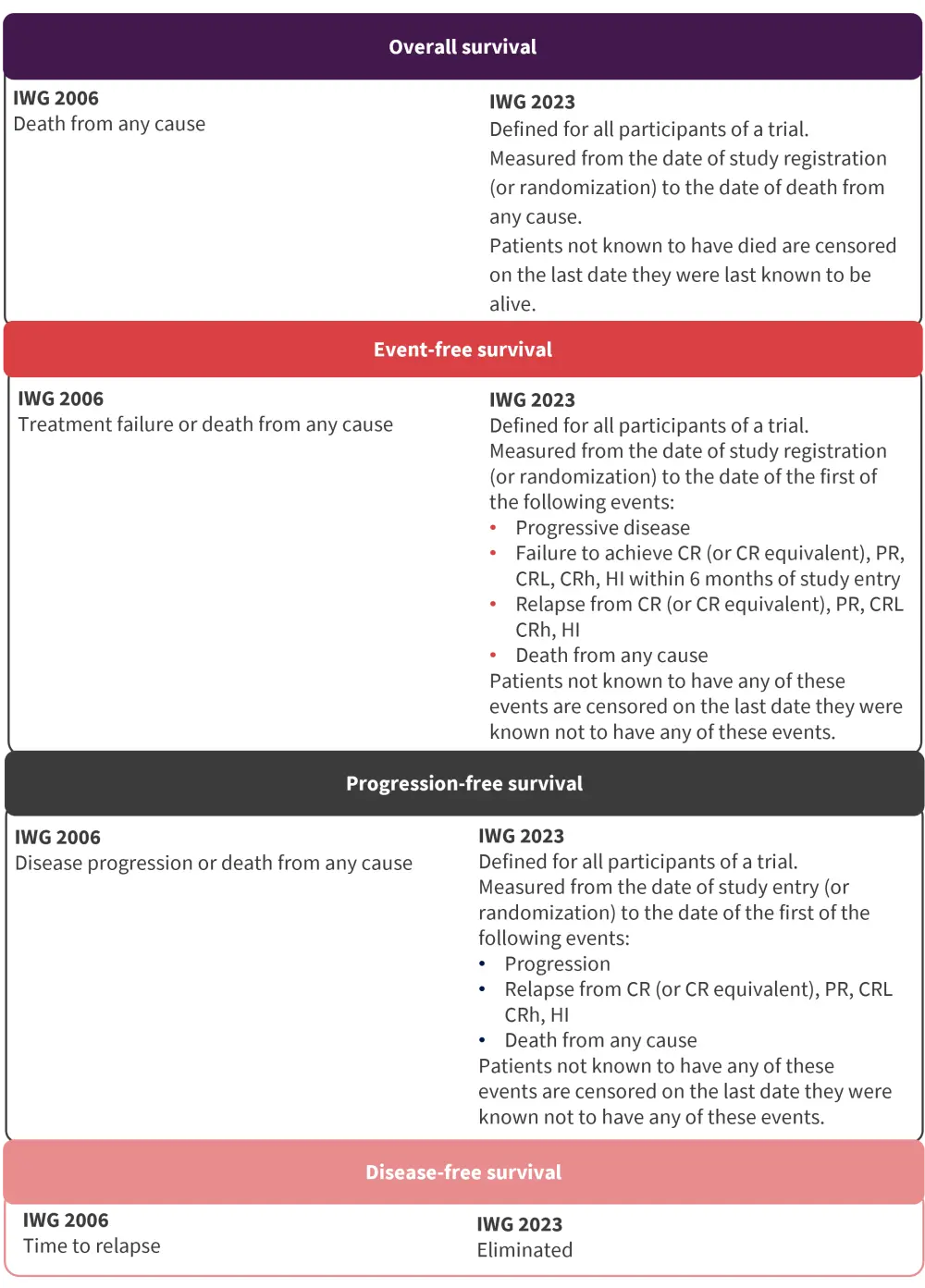
CR, complete remission; CRh, CR with partial hematologic recovery; CRL, CR with limited count recovery; HI, hematologic improvement; IWG, International Working Group; PR, partial remission.
*Adapted from Zeidan, et al.1
Patient-reported outcome endpoints
- Patient-reported outcome, defined as any report of the status of a patient’s health condition that comes directly from the patient without interpretation of the patient’s response by a clinician or anyone else, should be included as endpoints in phase II and phase III trials.
Conclusion
The 2023 IWG criteria represent a significant update compared with the 2006 and 2018 IWG criteria. The updated IWG criteria should lead to improved reporting of clinically relevant outcomes including the association between patient-centered outcomes and clinical trial results, reduced inconsistencies with AML response criteria, and improved applicability of novel therapies. Further research is required to elucidate the role of measurable residual disease assessment and to define other surrogate endpoints associated with OS. Molecular and less-than-CR responses need validation, and the proposed response criteria also require validation across different treatment settings.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

