All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Molecular analysis of TP53-mutant AML and MDS-EB
The presence of TP53 mutations in acute myeloid leukemia (AML) and myelodysplastic syndromes with excess blasts (MDS-EB) has been identified as a prognostic factor for poor survival outcomes and complex karyotypes. Despite this, a comparison of the molecular characteristics and treatment responses in patients with TP53-mutated AML and MDS-EB has not yet been performed. If mutant TP53 AML and MDS-EB were found to be similar with respect to molecular characteristics and survival, such findings could suggest a new classification of these disease subtypes as a single molecular disease entity.
An in-depth clinical and molecular analysis of TP53-mutated AML/MDS-EB was recently published by Grob et al.1 in Blood. We summarize key results below.
Methods
A total of 2,200 patients with newly diagnosed AML/MDS-EB who received standard induction chemotherapy were analyzed via next-generation sequencing (NGS).
The study design is summarized in Figure 1.
Figure 1. Study design*
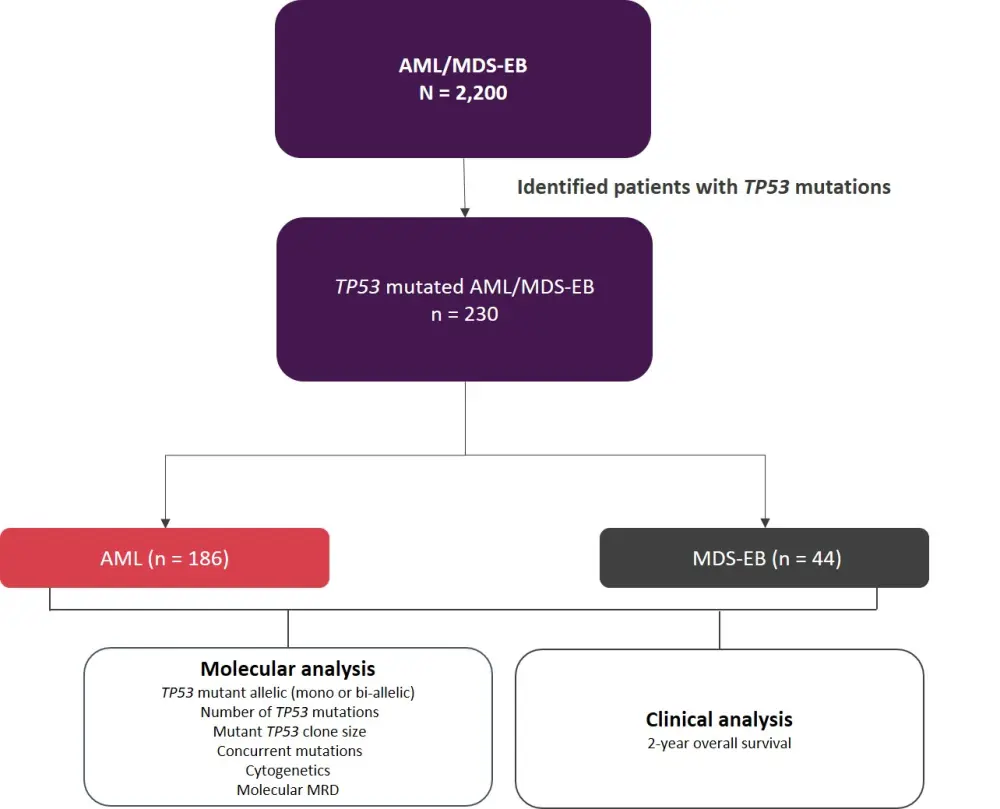
AML, acute myeloid leukemia; MDS-EB, myelodysplastic syndromes with excess blasts; MRD, minimal residual disease.
*Adapted from Grob et al.1
Results
Patient characteristics for the AML and MDS-EB cohorts are summarized in Table 1. No significant differences in patient characteristics were observed apart from a greater presence of deletion 5q in patients with MDS-EB (p = 0.025).
Table 1. Patient characteristics for AML and MDS-EB cohorts*
|
AML, acute myeloid leukemia; HSCT, hematopoietic stem cell transplantation; MDS-EB, myelodysplastic syndromes with excess blasts. |
||
|
Characteristic |
AML |
MDS-EB |
|---|---|---|
|
Median age, years (range) |
62 (18–80) |
63 (35–73) |
|
Female, % |
40 |
43 |
|
White blood cell count ≤100, % |
99 |
100 |
|
Allogeneic HSCT, % |
74 |
75 |
|
Cytogenetics, % |
||
|
Monosomy 5 |
28 |
27 |
|
Deletion 5q |
44 |
63 |
|
Monosomy 7 |
32 |
34 |
|
Monosomy 17 |
40 |
24 |
|
Abnormality 17p |
18 |
15 |
|
Complex karyotype |
83 |
90 |
|
Monosomal karyotype |
78 |
85 |
Molecular analysis
A molecular analysis of mutant allele status, number of TP53 mutations, TP53 variant allele frequency (VAF), and concurrent mutations are summarized in Table 2. No significant difference was reported in any of the molecular characteristics between TP53-mutated AML and MDS-EB. The most prevalent concurrent mutations reported in the entire AML/MDS-EB cohort included DNMT3A (13%), TET2 (9%), ASXL1 (5%), RUNX1 (5%), and SRSF2 (6%).
Most patients with AML/MDS-EB had complex karyotype (84%)
- The presence of complex karyotype was observed in 97% of patients with bi-allelic TP53-mutated AML/MDS-EB, 94% of patients with multiple TP53 mutations, and 94% of patients with larger TP53 clones (VAF >40%)
- Concurrent mutations were more prevalent in patients with a non-complex karyotype
Table 2. Molecular characterization of TP53-mutated AML/MDS-EB*
|
AML, acute myeloid leukemia; MDS, myelodysplastic syndromes; MDS-EB, myelodysplastic syndromes with excess blasts; VAF, variant allele frequency. |
|||
|
Characteristic |
AML |
MDS |
AML/MDS-EB |
|---|---|---|---|
|
TP53-mutant bi-allelic status, % |
77 |
68 |
76 |
|
Multiple TP53 mutations, % |
19 |
30 |
21 |
|
TP53 VAF, median (range) |
48 (1–97) |
41 (3–91) |
47 (1–97) |
|
Concurrent mutation, % |
51 |
41 |
49 |
Treatment outcomes
- Overall, the presence of TP53 mutations was associated with reduced overall survival (OS) when compared with wildtype patients (2-year OS, 12.8% vs 42.5%, respectively; p < 0.001).
- Notably, OS was comparable between TP53-mutated AML and MDS-EB (p = 0.549), and so further analysis grouped these diseases together.
- Evaluating the relationship of molecular characteristics with OS, mono- or bi-allelic TP53 demonstrated comparable poor survival (p = 0.327).
- Similarly, neither the number of TP53 mutations, rising VAF, or aberrations involving chromosome 17 had a significant impact on OS
- In contrast, the presence of concurrent mutations produced a small survival benefit (p = 0.042)
- Complex karyotype was associated with a reduced 2-year OS compared with non-complex karyotype (9% vs 34%; p = 0.002), which was not impacted by the type of consolidation therapy.
- OS in patients with non-complex karyotype TP53-mutated AML/MDS-EB was still poor
- The presence of wildtype TP53 appeared to improve survival outcomes in patients with complex karyotype when analyzing the whole cohort of 2,200 patients (p < 0.001).
Molecular minimal residual disease (MRD)
- As molecular MRD can predict survival outcomes, the authors sequenced bone marrow samples from 62 patients in morphologic complete remission.
- A total of 45 out of 62 patients had detectable TP53 mutations, but this was not associated with reduced OS (p = 0.653).
Conclusion
Overall, detailed molecular analysis of TP53-mutated AML/MDS-EB revealed homogeneity of molecular characteristics and clinical outcomes between these disease types. As such, the authors indicated a new classification of TP53-mutated AML/MDS-EB as a single disease entity.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

