All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Impact of lenalidomide discontinuation in patients with MDS and del(5q) achieving transfusion independence: HARMONY Alliance study
Do you know... In a retrospective study by Crisá, which of the following factors was not found to be significantly associated with EFS in patients with MDS and del(5q) treated with lenalidomide?
Lenalidomide demonstrates successful clinical activity in patients with myelodysplastic syndrome (MDS) who harbor deletion 5q (del[5q]) and has been shown to achieve red blood cell transfusion independence (RBC-TI) as well as complete cytogenetic response (CCyR). However, lenalidomide is associated with high treatment costs, serious side effects such as thromboembolism or cytopenia, and impacts on patients’ quality of life.1 The MDS Hub has previously covered several articles on lenalidomide.
During the European Hematology Association (EHA) 2023 congress, Elena Crisá presented the findings from the HARMONY Alliance investigating the impact of lenalidomide discontinuation in patients with MDS and del(5q) after achieving RBC-TI.1 We are pleased to summarize the findings here.
Study design
This is a retrospective cohort study in patients aged ≥18 years, diagnosed with MDS with isolated del(5q) or a del(5q) with one additional abnormality (excluding chromosome 7 abnormalities), and who had achieved RBC-TI with lenalidomide. Patients who discontinued lenalidomide due to lack of response or disease progression, as well as those with transfusion-dependent anemia at lenalidomide stop, were excluded.
The primary endpoint was event-free survival (EFS). Secondary endpoints included:
- RBC-TI duration
- Overall survival
- Progression-free survival
- Time to acute myeloid leukemia
- Response rate to lenalidomide re-challenge
- Biological and clinical predictors of response duration after discontinuation
Results
A total of 115 patients were included with a median age of 77 years (range, 42–93 years). The median follow-up period was 40 months (range, 2–213 months), and the median number of lenalidomide cycles was 12 (range, 1–72 cycles). Patient characteristics are shown in Table 1.
Table 1. Patient characteristics*
|
IPSS, International Prognostic Scoring System; IPSS-R, Revised International Prognostic Scoring System; MDS, myelodysplastic syndromes; MDS-EB, MDS with excess blasts; MDS-MLD, MDS with multilineage dysplasia; RA, refractory anemia; RAEB-I, refractory anemia with excess blasts-1; RAEB-II, refractory anemia with excess blasts-2; RBC, red blood cell; RCMD, refractory cytopenia with multilineage dysplasia. |
|
|
Characteristic |
Total patients (N = 115), % |
|---|---|
|
Sex |
|
|
Female |
80.9 |
|
Male |
19.1 |
|
WHO 2008 |
|
|
RA |
0.9 |
|
RCMD |
1.7 |
|
MDS with 5Q syndrome |
87.0 |
|
RAEB-I |
9.6 |
|
RAEB II |
0.9 |
|
WHO 2016 |
|
|
MDS-MLD |
0.9 |
|
MDS-EB |
10.4 |
|
MDS with isolated del(5q) |
88.7 |
|
Therapy related |
|
|
No |
95.6 |
|
Yes |
4.4 |
|
IPSS |
|
|
Low |
54.9 |
|
Intermediate I |
45.1 |
|
IPSS-R |
|
|
Very low |
26.0 |
|
Low |
51.0 |
|
Intermediate I |
22.9 |
|
RBC-transfusion dependence (>2 units in 8 weeks) |
|
|
No |
17.4 |
|
Yes |
82.6 |
Toxicity
Lenalidomide dose reduction due to toxicity was required in 40% of patients. In addition, in most patients who discontinued lenalidomide, this was due to toxicity (Figure 1).
Figure 1. Reasons for lenalidomide discontinuation*
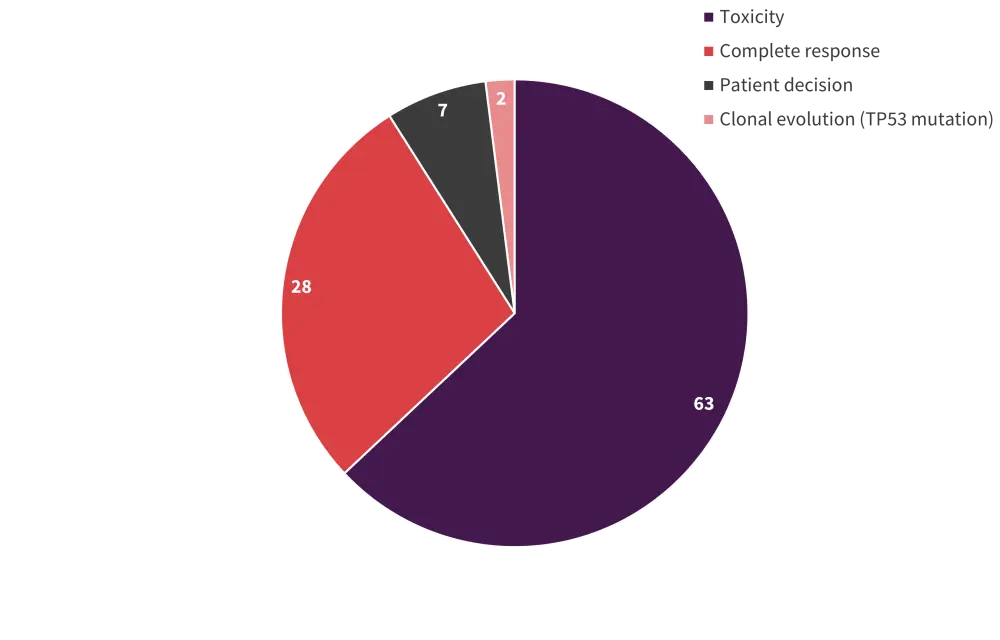
*Adapted from Crisá E.1
EFS after lenalidomide discontinuation
At the time of lenalidomide discontinuation, 115 patients had RBC-TI and 90 patients achieved a cytogenetic response. Overall, CCyR and partial CyR were achieved in 48.9% and 66.6% of patients, respectively. At a median duration of 34.6 months, 48% of patients achieved EFS. A total of 40.9% of patients lost RBC-TI, and 73% of patients lost CCyR. Progression to high-risk MDS, acute myeloid leukemia, clonal evolution, or chronic myelomonocytic leukemia-2 occurred in 21% of patients, and 38.3% patients died.
There was a significant association between EFS and transfusion dependence at the start of lenalidomide treatment (p = 0.008) and the number of lenalidomide cycles received (p < 0.001) (Figures 2A and 2B).
- The median duration of EFS was 79 months in patients who started lenalidomide earlier and had achieved RBC-TI versus 31 months in those who started lenalidomide later and were transfusion-dependent.
- The median duration of EFS was 58 months in patients who received >12 cycles versus 18 months in those who received ≤12 cycles of lenalidomide.
Figure 2A and 2B. Factors impacting EFS*
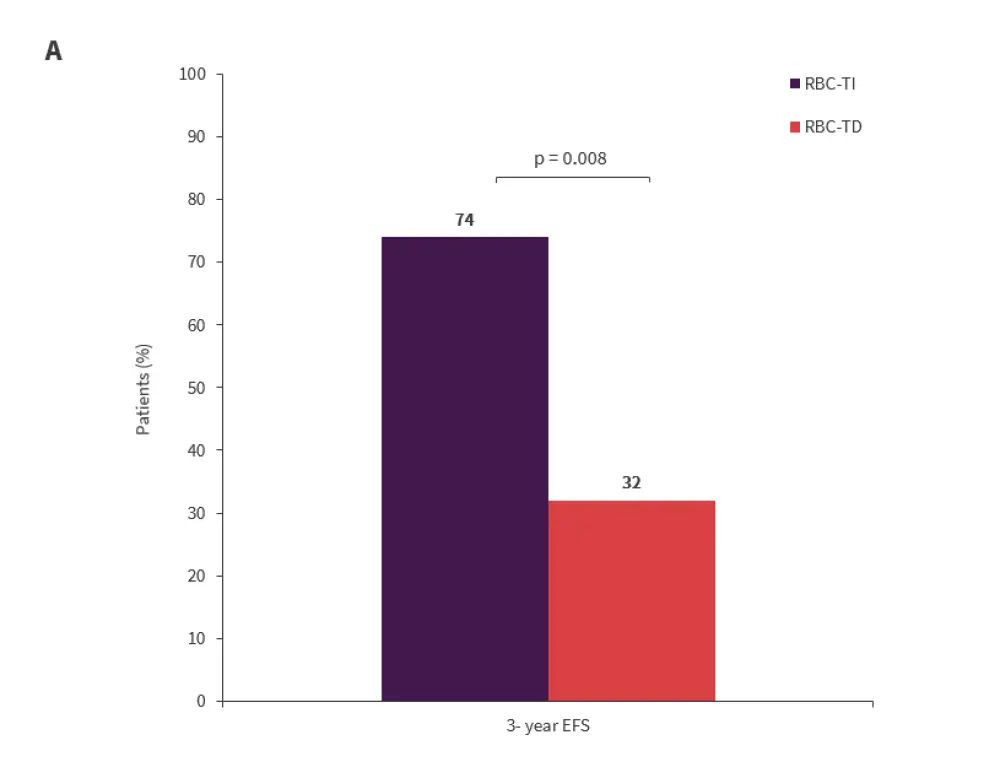
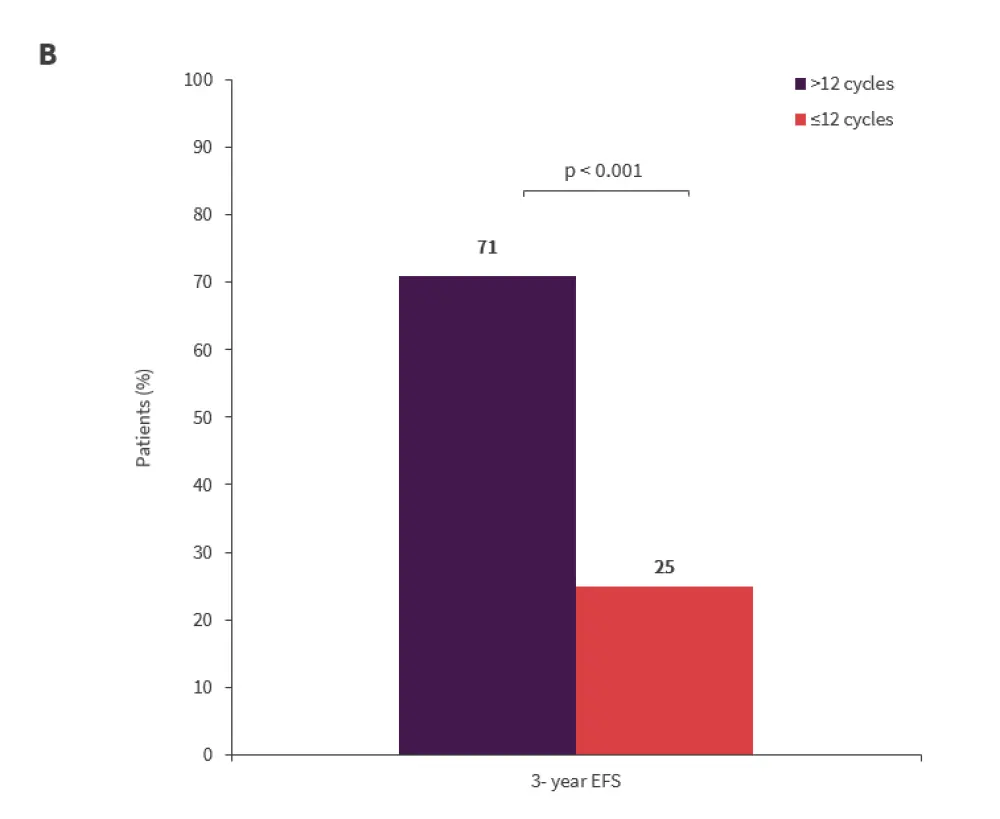
*Adapted from Crisá E.1
EFS, event-free survival; RBC-TD, red blood cell transfusion dependent; RBC-TI, red blood cell transfusion independent.
The multivariate analysis showed that the following factors were significantly associated with EFS:
- RBC-TD versus RBC-TI (hazard ratio [HR], 2.919; p = 0.032)
- Revised International Prognostic Scoring System very low versus low/intermediate status (HR, 0.436; p = 0.027)
- Lenalidomide cycles <12 versus >12 (HR, 4.360; p < 0.001).
RBC-TI duration after lenalidomide discontinuation
The median RBC-TI duration after lenalidomide discontinuation was 56 months. Patients who achieved CCyR, received >12 cycles of lenalidomide, and achieved RBC-TI showed longer duration of RBC-TI (Table 2).
The median duration of survival was shorter in patients who lost RBC-TI compared with those who retained RBC-TI (71 vs 102 months; p = 0.029). Of the 46 patients re-treated with lenalidomide for response loss, 65% (26/40) of the evaluable patients achieved at least a hematological response, including 3 patients who achieved a CCyR.
Table 2. RBC-TI duration after lenalidomide discontinuation*
|
CCyR, complete cytogenetic response; NR, not reached; RBC-TD, red blood cell transfusion dependence; RBC-TI, red blood cell transfusion independence. |
||
|
Factors |
Median duration of RBC-TI, months |
RBC-TI at 3 years, % |
|---|---|---|
|
CCyR (n = 44) |
86 |
77 |
|
No CCyR (n = 46) |
86 |
49 |
|
> 12 cycles (n = 54) |
NR |
87 |
|
≤ 12 cycles (n = 61) |
86 |
39 |
|
RBC-TI (n = 20) |
NR |
85 |
|
RBC-TD (n = 95) |
44 |
58 |
Conclusion
This retrospective study presented by Elena Crisá demonstrates that there is potential to stop lenalidomide after achieving RBC-TI, particularly after at least 12 months of treatment, while limiting toxicities, reducing utilization of healthcare, and improving patients’ quality of life. The authors hope that future studies will validate the findings of this study.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

