All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Expert recommendations for clinical trial design to facilitate drug development in MDS
There are several challenges in developing myelodysplastic syndrome (MDS) clinical trials, including defining positive treatment outcomes, unclear response criteria, inflexibility of transfusion thresholds, and difficulty validating patient-reported outcomes.
Sekeres et al.1 recently published expert recommendations for improving clinical trial design and endpoints to facilitate the development of novel therapies in MDS in Clinical Cancer Research. The MDS Hub is pleased to summarize the recommendations below.
Recommendations for trial designs
Figure 1. Recommendations for trial designs*

HMA, hypomethylating agents
*Data from Sekeres, et al.1
Several further recommendations were suggested that should be considered, irrespective of clinical trial phases (Figure 2).
Figure 2. Recommendations for all phases of MDS trial designs*
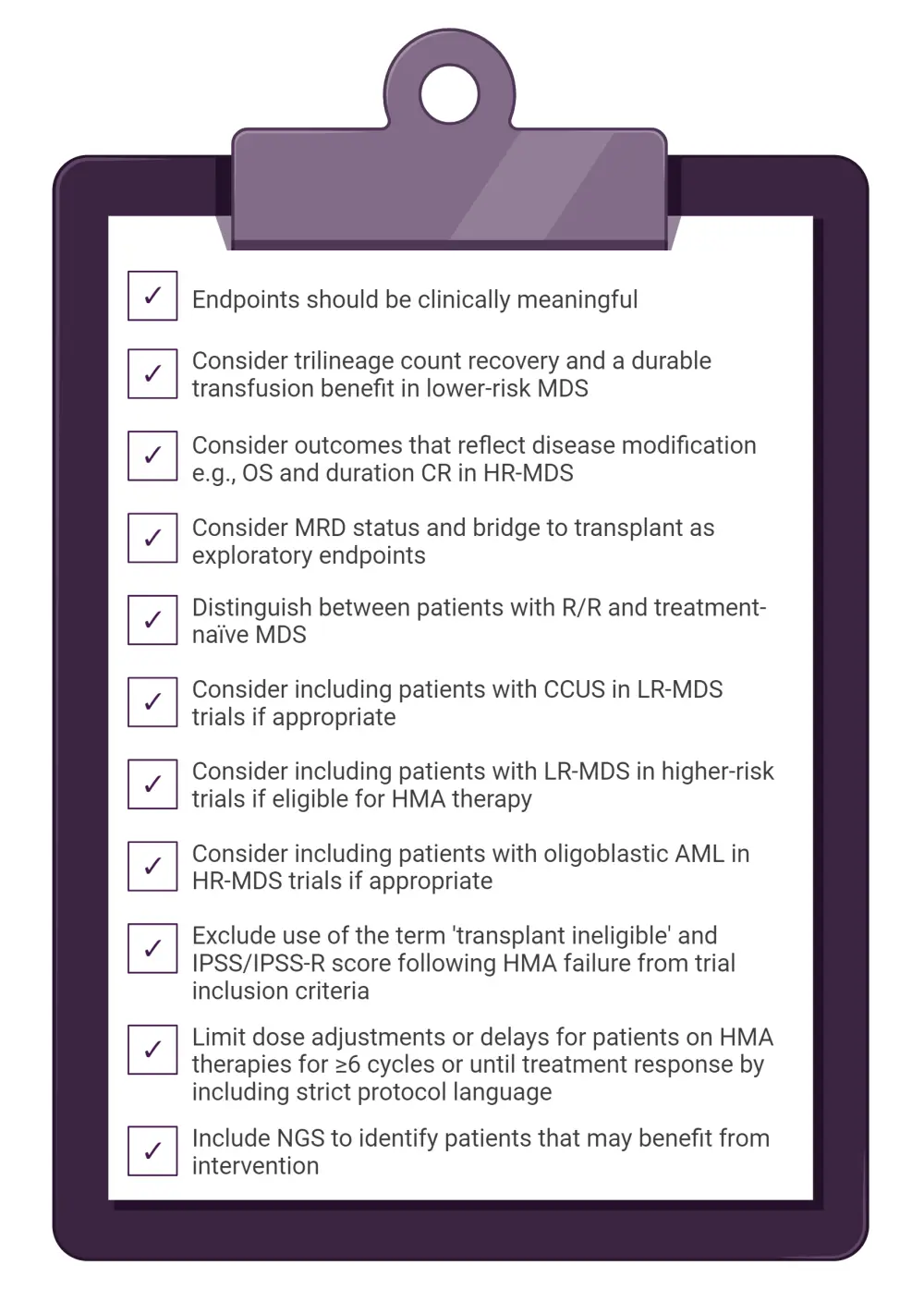
AML, acute myeloid leukemia; CCUS, clonal cytopenia of undetermined significance; CR, complete response; HMA, hypomethylating agent; HR-MDS, higher-risk myelodysplastic syndrome; IPSS, International Prognostic Scoring System; IPSS-R, Revised IPSS; LR-MDS, lower-risk myelodysplastic syndrome; MDS, myelodysplastic syndrome; MRD, minimum disease residual; NGS, next-generation sequencing; OS, overall survival; R/R, relapsed/refractory.
*Data from Sekeres, et al.1
Recommendations for developing clinically meaningful endpoints
Determining a clinically meaningful endpoint depends on the risk status of the patient. However, in all MDS subtypes, there is a significant improvement in the patient’s quality of life (QoL), and these improvements translate into meaningful outcomes in real-world care settings.
Lower-risk MDS
- Endpoints should consider managing cytopenia, preventing progression, and improving overall survival (OS)
- Response criteria for patients with anemia should be based on baseline transfusion dependency, improvements in symptom burden, and QoL
- Patient-reported outcomes may help capture the impact of treatment from the patient’s perspective
- Clinical trials evaluating targeted therapies should assess cytogenetic and molecular responses by measuring minimal residual disease
- OS is an appropriate endpoint in patients with low-risk disease features with adverse risks such as EZH2 (enhancer of zeste homolog 2), RUNX1 (runt-related transcription factor 1), tumor protein p53, and ASXL1 (ASXL transcriptional regulator 1)
- OS may be used as a critical safety endpoint in randomized trials to ensure there is no increase in mortality due to toxicities.
Higher-risk MDS
- Primary endpoints should consider modification of the disease
- Endpoints should be clear, simple to interpret, and focus on OS and durable responses
- Trials should prospectively evaluate the feasibility of alternative complete response definitions
- Overall response rates should be limited to true complete response, as well as partial response and hematologic improvement
- Patient-reported outcomes should be utilized to determine QoL and symptom improvement
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

