All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Eltrombopag for patients with LR-MDS and thrombocytopenia: Results from the EQOL-MDS trial
Do you know... What type of therapeutic agent is eltrombopag?
Severe thrombocytopenia (<30 × 103/mm3 platelets [PLTs]) occurs in ≥10% of patients with lower-risk myelodysplastic syndromes (LR-MDS) and is associated with inferior survival outcomes.1 The current standard of care for thrombocytopenic patients with LR-MDS is PLT transfusion.1 Eltrombopag is an oral, small-molecule thrombopoietin receptor agonist,1 previously covered by the MDS Hub.
In 2017, Oliva et al.2 published short-term outcomes from the phase II EQOL-MDS trial (NCT02912208) where eltrombopag was associated with improved PLT response versus placebo and an acceptable safety profile in patients with LR-MDS and severe thrombocytopenia.2 Recently, Oliva et al.1 published the interim long-term safety and efficacy results from this trial in the Journal of Clinical Oncology, which we are pleased to summarize here.
Study design and patient characteristics
The multicenter, randomized, single-blind, placebo-controlled, phase II EQOL-MDS trial assessed the safety and efficacy of eltrombopag versus placebo in 169 patients with LR-MDS (Figure 1). In total, 165 patients received ≥1 dose of the study drug (eltrombopag, n = 111; placebo, n = 54) and were included in the modified intention-to-treat analysis. Baseline characteristics were comparable between treatment arms. The data cutoff for this analysis was March 3, 2022.
Figure 1. EQOL-MDS study overview*
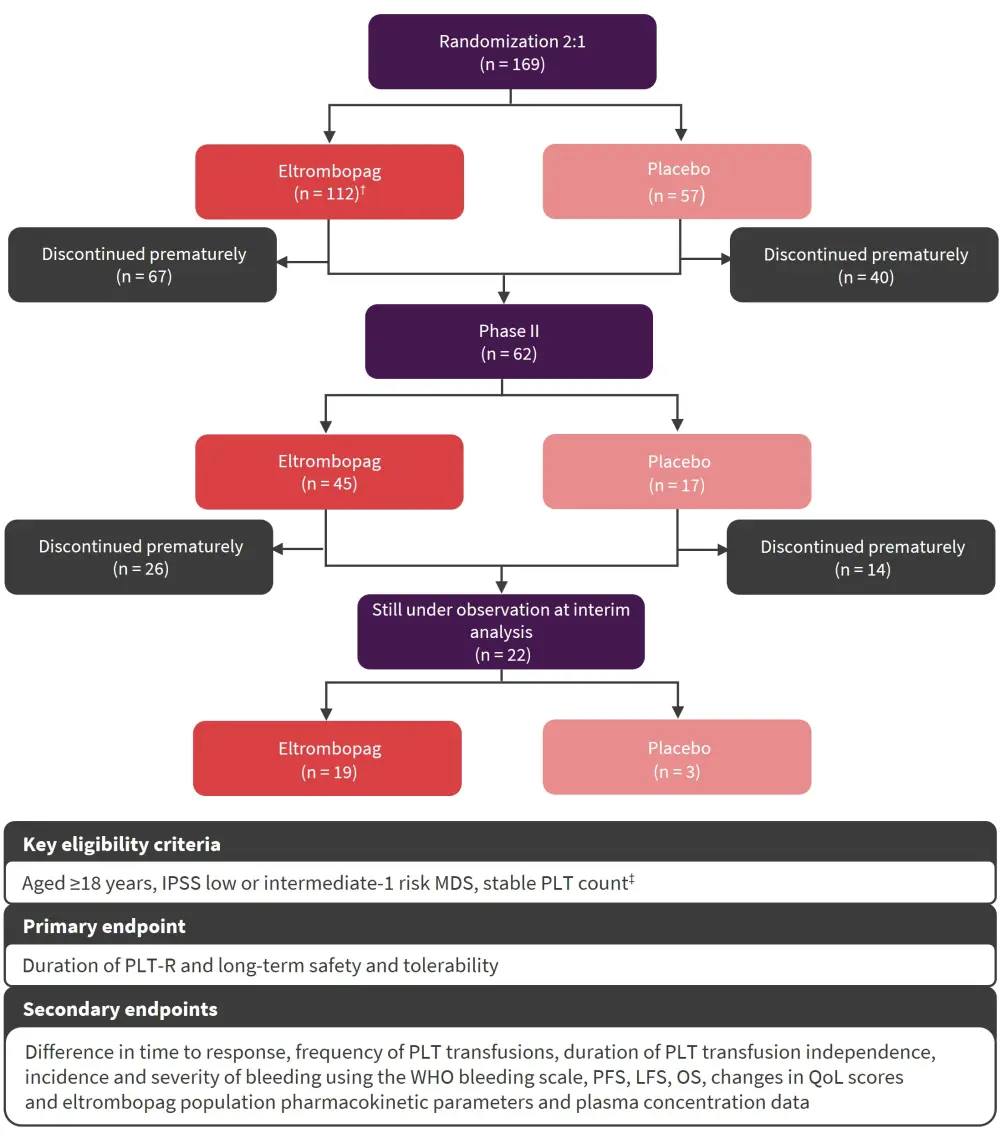
IPSS, International Prognostic Scoring System; LFS, leukemia-free survival, MDS, myelodysplastic syndromes; OS, overall survival; PFS, progression-free survival; PLT, platelet; PLT-R, PLT response; QoL, quality of life; WHO, World Health Organization.
*Adapted from Oliva, et al.1
†50 mg/day, titrated in 50 mg increments every 2 weeks up to 300 mg.
‡Defined as <30 × 103/mm3 without exceed >200 Gi/L.
Key findings
PLT response
At a median follow-up time of 25 weeks (interquartile range [IQR], 14–68), eltrombopag was associated with a greater PLT response compared with placebo (odds ratio, 5.9; p < 0.001; Figure 2).
Figure 2. PLT response*
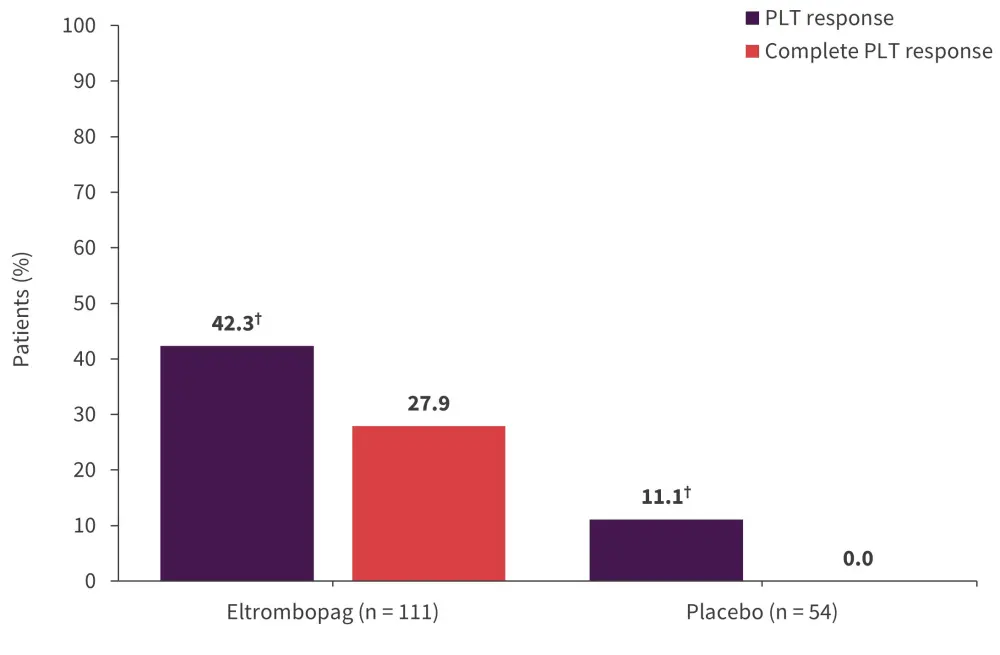
PLT, platelet.
*Adapted from Oliva, et al.1
†p < 0.001.
The median daily drug dose at response was 50 mg (IQR, 50–150). In the eltrombopag arm, the median PLT increment at best response in the first 24 weeks was significantly higher (31 × 103/mm3; IQR, 8–103) than in the placebo arm (9.5 × 103/mm3; IQR, 1.75–17; p < 0.0001).
In the multivariate analysis, only baseline hemoglobin levels were significantly associated with PLT response (hazard ratio, 1.21; p = 0.003). The median duration of response in the overall population was not reached. The cumulative thrombocytopenia relapse-free survival in the eltrombopag arm at 60 months was 63.6% (95% confidence interval, 46.0–81.2). The PLT response duration between responders in the eltrombopag arm and the placebo arm was not significantly different (p = 0.14).
Safety
Safety analysis at the time of data cutoff revealed:
- 9.9% and 7.4% of patients experienced unrelated deaths in the eltrombopag and placebo arms, respectively.
- 62 patients in the eltrombopag arm experienced Grade 1–2 adverse events compared with 29 in the placebo arm (exposure-adjusted incidence rate, 0.76; p = 0.23).
- Grade 3–4 non-hematologic adverse events occurred in 50 versus 11 patients in the eltrombopag and placebo arm (exposure-adjusted incidence rate, 2.5 vs 1.6).
- Clinically significant bleeding was experienced by 31.5% versus 19.8% of patients in the placebo and eltrombopag arm, respectively (incidence rate ratio, 0.54; p = 0.0002).
- 18 patients in the eltrombopag arm discontinued treatment due to severe liver or persistent Grade 3–4 adverse events versus 0 patients in the placebo arm.
PLT transfusion independence
Of the PLT transfusion-dependent patients, 54.3% of patients in the eltrombopag arm versus 35.3% in the placebo arm achieved PLT transfusion independence (p = 0.24). The median duration of PLT transfusion was 32 weeks versus 9 weeks in the eltrombopag and placebo arm, respectively (p = 0.15). The median time to PLT response was 2.1 weeks (range, 1–6) in the eltrombopag arm and 54 weeks (range, 6–155; p < 0.001) in the placebo arm.
Progression of disease and survival outcomes
In total, 8% of patients in the eltrombopag arm experienced progression of MDS compared with 9% of patients in the placebo arm (p = 0.774). In the eltrombopag arm, 9% of patients progressed to acute myeloid leukemia after a median time from start of treatment of 13 weeks compared with 7% of patients in the placebo arm (p = 0.729) with a median time from start of treatment of 34 weeks. Overall, acute myeloid leukemia evolution and/or disease progression was 17% in both the eltrombopag arm and the placebo arm (p = 1.0). The 5-year cumulative leukemia-free survival, progression-free survival, combined outcome-free survival, and overall survival were not significantly different between the eltrombopag and placebo arms (Figure 3).
Figure 3. Survival outcomes*
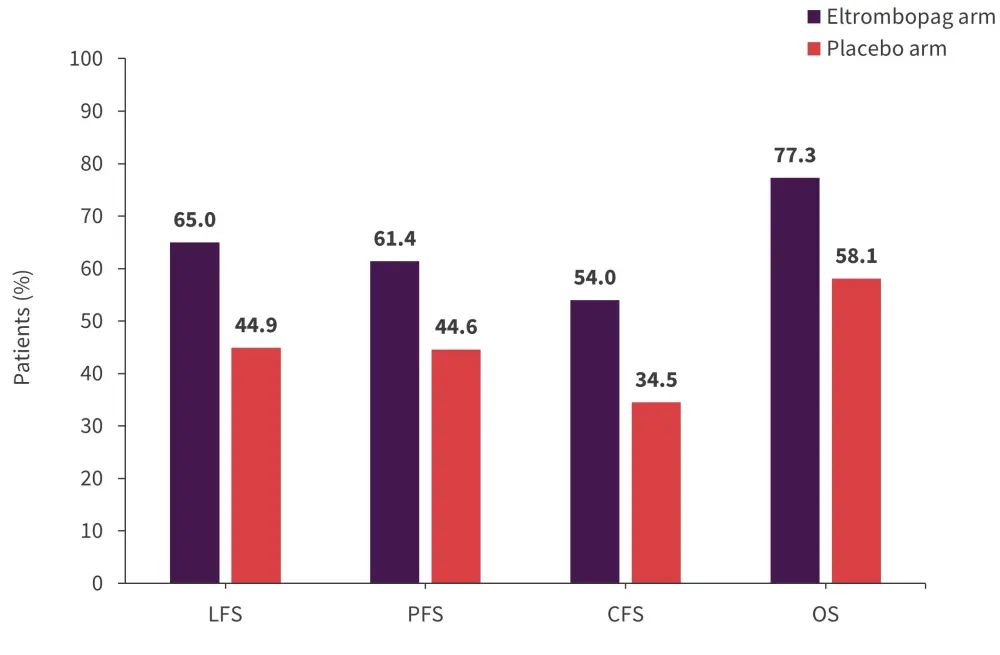
CFS, combined outcomes-free survival; LFS, leukemia-free survival; OS, overall survival; PFS, progression-free survival.
*Data from Oliva, et al.1
Conclusion
This interim analysis from the EQOL-MDS study confirmed the results from the previous short-term analysis. This indicates that eltrombopag is associated with an increased and durable PLT response versus placebo in adult patients with LR-MDS and severe thrombocytopenia at a relatively low optimal dose of 50 mg once daily. Eltrombopag had an acceptable safety profile, and patients who received eltrombopag increased and maintained their PLT count and experienced a reduction in bleeding events without an associated risk of MDS progression. Further studies may confirm the benefit of eltrombopag in larger populations.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


