All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Conditioning regimens prior to allo-HSCT: Updates from the 48th Annual Meeting of the EBMT (2022)
Do you know... When used for adult patients with AML in CR1/CR2, fludarabine in combination with total body irradiation (TBI) at 12 Gy (FluTBI12) compared with the standard conditioning regimen cyclophosphamide plus TBI at 12 Gy (CyTBI12) is statistically significantly associated with which of the following?
The standard myeloablative conditioning regimen (MAC) for patients with acute myeloid leukemia (AML) undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT) is busulfan (BUS) or total body irradiation (TBI) in combination with cyclophosphamide (Cy). More recently, fludarabine (Flu) has been investigated as an alternative to cyclophosphamide to lessen regimen toxicity. Treosulfan, an analogue to busulfan, has also been investigated as an alternative to improve efficacy.
At the 48th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT), the safety and efficacy of conditioning combinations were evaluated for patients undergoing allo-HSCT, using data from the Acute Leukemia Working Party (ALWP) of the EBMT. We summarize results from four key talks below.
TBI plus fludarabine versus busulfan plus fludarabine1
Ryszard Swoboda presented a retrospective study1 comparing a combination of fludarabine with the standard myeloablative regimens of either TBI at 12 Gy (FluTBI12) or intravenous busulfan (FB4) before allo-HSCT (Figure 1).
Figure 1. Study design*
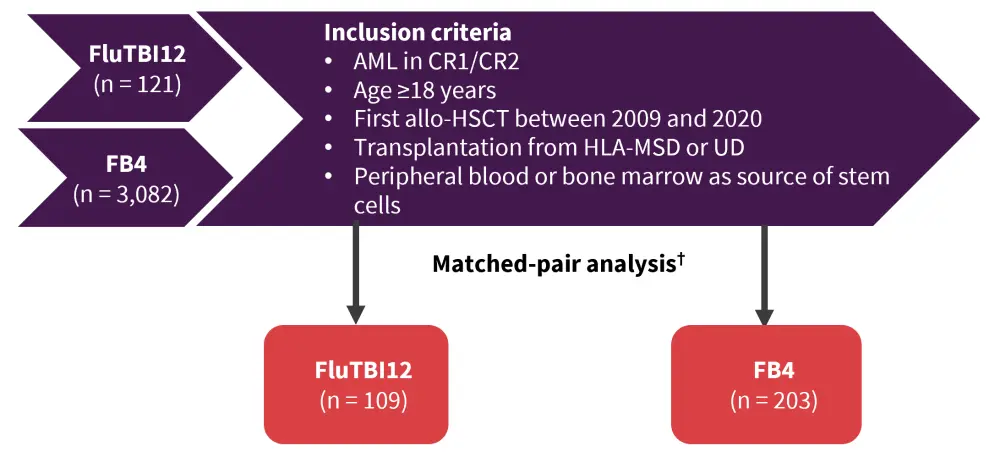
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; CR1, first complete remission; CR2, second complete remission; FB4, busulfan intravenous at a dose of 12.8 mg/kg (4 days) plus fludarabine; FluTBI12, fludarabine with total body irradiation at a dose of 12 Gy; HLA, human leukocyte antigen; MSD, matched sibling donor; UD, unrelated donor.
*Adapted from Swoboda et al.1
†Patients were exactly matched for cytogenetic risk groups, disease status at allo-HSCT (CR1/CR2), donor type (MSD, UD), and stem cell source (peripheral blood/bone marrow).
Results
- Patient characteristics are summarized in Table 1; there were no significant differences apart from serological status with patients receiving FB4 more frequently positive for cytomegalovirus (p = 0.0004).
Table 1. Patient characteristics*
|
CR1, first complete remission; FB4, busulfan intravenous at a dose of 12.8 mg/kg (4 days) plus fludarabine; FluTBI12, fludarabine with total body irradiation at 12 Gy; HLA, human leukocyte antigen. |
||
|
Characteristic |
FluTBI12 |
FB4 |
|---|---|---|
|
Median age, years (range) |
41 (18.2–61.8) |
41.4 (18.7–67.4) |
|
Male/Female, % |
61.5/38.5 |
62.4/37.6 |
|
Cytogenetic risk group, % |
||
|
Standard |
8.3 |
8 |
|
Intermediate |
41.3 |
42.3 |
|
High |
11.9 |
11.7 |
|
Unknown |
38.5 |
38 |
|
In CR1 at transplantation, % |
78 |
78.9 |
|
Median year of transplantation (range) |
2017 (2009–2020) |
2016 (2009–2020) |
|
Donor type, % |
||
|
HLA-identical sibling |
32.4 |
27.7 |
|
10/10 HLA-matched unrelated |
54.1 |
51.4 |
|
9/10 HLA-mismatched unrelated |
13.5 |
20.8 |
- Survival outcomes are shown in Table 2. Notably, no significant differences were reported between the conditioning regimens for any of the efficacy and toxicity parameters.
Table 2. Efficacy and toxicity results of FluTBI12 versus FB4*
|
FB4, busulfan intravenous at a dose of 12.8 mg/kg (4 days) plus fludarabine; FluTBI12, fludarabine with total body irradiation at 12 Gy; GvHD, graft-versus-host disease. |
|||
|
Parameter, % |
FluTBI12 |
FB4 |
HR (95% CI) |
|---|---|---|---|
|
Relapse incidence |
19.2 |
29.4 |
1.55 (0.9–2.66) |
|
Nonrelapse mortality |
15.6 |
10.9 |
0.62 (0.34–1.15) |
|
Leukemia-free survival |
65.2 |
59.8 |
1.1 (0.73–1.67) |
|
Overall survival |
69.7 |
72.1 |
0.96 (0.61–1.52) |
|
GvHD-free, relapse-free survival |
48.5 |
48.5 |
0.95 (0.67–1.36) |
|
Acute GvHD grade |
|||
|
2–4 |
17.8 |
24.4 |
1.49 (0.86–2.6) |
|
3–4 |
7.5 |
6 |
0.83 (0.34–2.07) |
|
Chronic GvHD |
41.8 |
34.3 |
0.77 (0.52–1.15) |
|
Extensive chronic GvHD |
16.4 |
15.9 |
0.96 (0.52–1.78) |
In summary, FluTBI12 and FB4 produced comparable efficacy and toxicity in adult patients receiving allo-HSCT. Swoboda et al.1 highlighted that the regimen choice should be guided based on patient history, availability of TBI, and experience of the centers using MAC.
Fludarabine plus TBI versus cyclophosphamide plus TBI2
Sebastian Giebel presented results from a retrospective study2 comparing the safety and efficacy of FluTBI12 versus cyclophosphamide plus TBI at 12 Gy (CyTBI12).
The study design is depicted in Figure 2.
Figure 2. Study design*
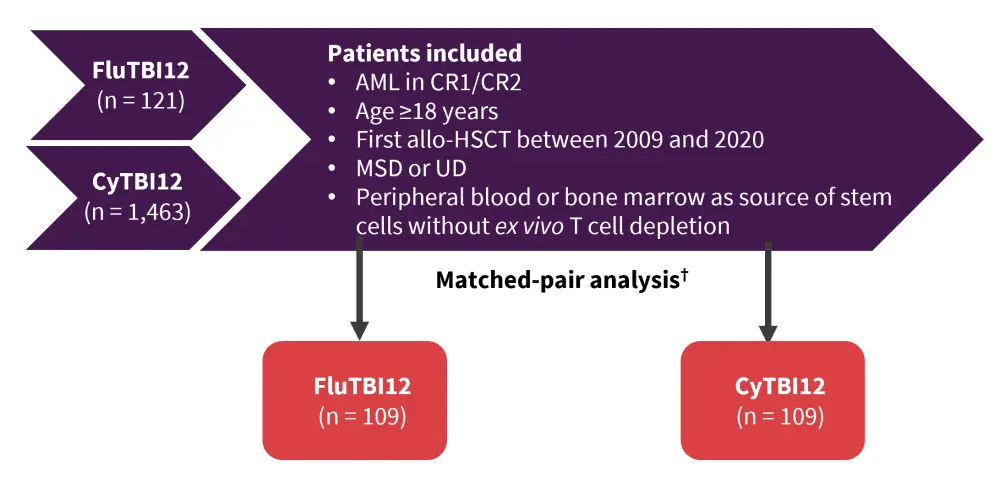
allo-HSCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; CR1, first complete remission; CR2, second complete remission; CyTBI12, cyclophosphamide plus total body irradiation at 12 Gy; FluTBI12, fludarabine plus total body irradiation at 12 Gy; MSD, matched sibling donor; UD, unrelated donor.
*Adapted from Giebel et al.2
†Patients were exactly matched for cytogenetic risk groups, disease status at allo-HSCT (CR1/CR2), donor type (MSD, UD), and stem cell source (peripheral blood/bone marrow).
Results
Patient characteristics are shown in Table 3. The only significant difference was found between the median year of transplantation (p < 0.0001).
Table 3. Patient characteristics*
|
CR1, first complete remission; CyTBI12, cyclophosphamide plus total body irradiation at 12 Gy; FluTBI12, fludarabine with total body irradiation at 12 Gy; HLA, human leukocyte antigen. |
||
|
Characteristic |
FluTBI12 |
CyTBI12 |
|---|---|---|
|
Median age, years (range) |
38 (18–69) |
40 (18–60) |
|
Cytogenetic risk group, % |
||
|
Standard |
8 |
8 |
|
Intermediate |
35 |
35 |
|
High |
13 |
13 |
|
Unknown |
44 |
44 |
|
In CR1 at transplantation, % |
78 |
78 |
|
Median year of transplantation (range) |
2016 (2009–2020) |
2012 (2009–2020) |
|
Donor type, % |
||
|
HLA-identical sibling |
37 |
32.5 |
|
10/10 HLA-matched unrelated |
51 |
54 |
|
9/10 HLA-mismatched unrelated |
11 |
14 |
There was no significant difference in leukemia-free survival (LFS), overall survival (OS), relapse incidence, nonrelapse mortality (NRM), chronic graft-versus-host disease (GvHD), or GvHD-free/relapse-free survival (GRFS) between conditioning regimens (Figure 3).
Figure 3. Survival outcomes*
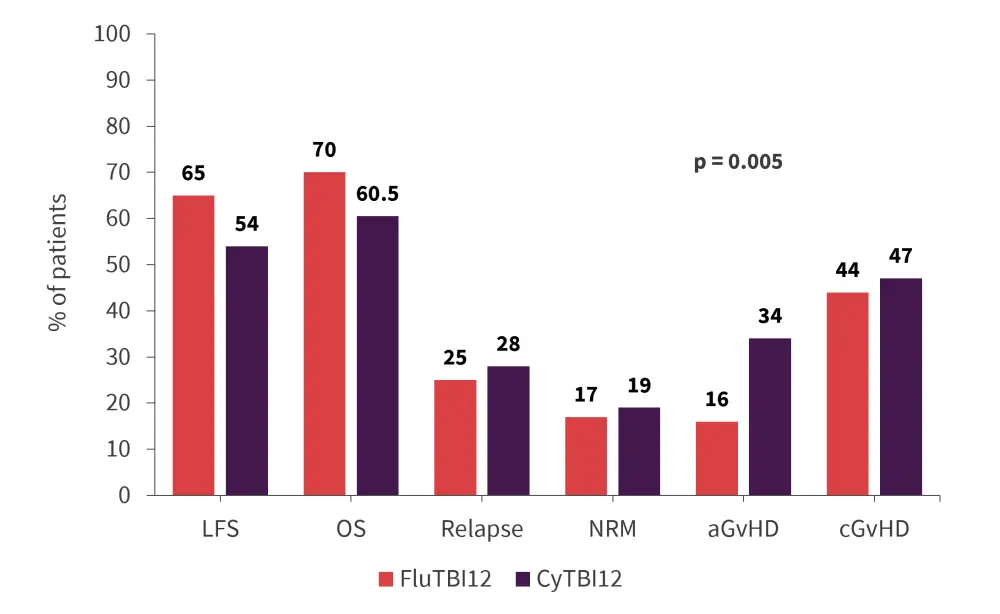
aGvHD, acute graft-versus-host disease; cGvHD, chronic graft-versus-host disease; CyTBI12, cyclophosphamide plus total body irradiation at 12 Gy; LFS, leukemia-free survival; FluTBI12, fludarabine plus total body irradiation at 12 Gy; OS, overall survival; NRM, nonrelapse mortality.
*Adapted from Giebel et al.2
There was a significant difference found in acute GvHD (aGvHD) incidence with patients receiving CyTBI12 experiencing higher rates of aGvHD (p = 0.005).
In summary, the data demonstrate that fludarabine is a valid substitute for cyclophosphamide in TBI containing conditioning regimens, with encouraging LFS and OS rates and, importantly, a lower incidence of Grade 2–4 aGvHD.
Treosulfan + fludarabine versus busulfan + fludarabine3
Treosulfan is an analogue of busulfan that has shown favorable outcomes when substituted for its counterpart in patients with AML/myelodysplastic syndromes (MDS) at mixed doses. However, conditioning with FB4 (fludarabine at 150 or 160 mg/m2 and busulfan at 12.8 mg/kg) was superior to FT14 (fludarabine at 150 or 160 mg/m2 and treosulfan at 42 g/m2) in younger patients (<55 years) with AML in complete remission. Eleni Gavriilaki presented results from a retrospective study3 comparing FB4 with FT14 in patients with relapsed/refractory AML. The study design is summarized in Figure 4.
Figure 4. Study design*
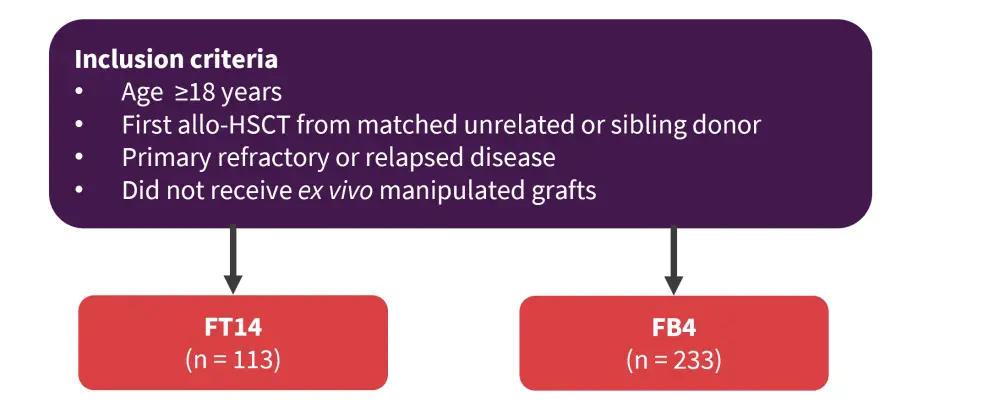
Allo-HSCT, allogeneic hematopoietic stem cell transplant; FB4, fludarabine at 150 or 160 mg/m2 and busulfan at 12.8 mg/kg; FT14, fludarabine at 150 or 160 mg/m2 and treosulfan at 42 g/m2.
*Adapted from Gavriilaki et al.3
Results
Patient characteristics are shown in Table 4.
Table 4. Patient characteristics*
|
AML, acute myeloid leukemia; FB4, fludarabine-busulfan; FT14, fludarabine-treosulfan; HSCT, hematopoietic stem cell transplant; MSD, matched sibling donor; UD, unrelated donor. |
||
|
Characteristic |
FT14 |
FB4 |
|---|---|---|
|
Median age, years (range) |
58.1 (21.2–75.9) |
52.4 (18.5–70.7) |
|
Median follow-up, months |
32.93 |
24.91 |
|
Adverse cytogenetics, % |
23 |
16.7 |
|
Time diagnosis to HSCT, median in months (range) |
6.9 (0.5–147.2) |
5.9 (0.3–66.2) |
|
Cell source, % |
||
|
Bone marrow |
3.5 |
9 |
|
Peripheral blood |
96.5 |
91 |
|
Diagnosis, % |
||
|
De novo |
69 |
78.5 |
|
Secondary AML |
31 |
21.5 |
|
Type of donor, % |
||
|
MSD |
31 |
44.6 |
|
UD |
69 |
55.4 |
|
Median dose of fludarabine, mg/m2 |
150 |
160 |
- The follow-up period and time of diagnosis to HSCT had a trend toward significant difference between the two cohorts.
- There was a significant difference in patient age (p = 0.0006), diagnosis (p = 0.054), type of donor (p = 0.015), and median dose of fludarabine between the groups (p < 0.0001).
Survival outcomes
- There was no significant difference in the cumulative incidence of aGvHD (FT14, 8.5% vs FB4, 9%), chronic GvHD (FT14, 10.7% vs FB4, 10.9%), and NRM (FT14, 20.8% vs FB4, 22.6%).
- In a multivariate analysis, treosulfan was demonstrated to be associated with improved LFS, relapse incidence, OS, and GRFS (Table 5).
Table 5. Multivariate analysis*
|
FB4, fludarabine-busulfan; FT14, fludarabine-treosulfan. |
||||||||
|
Factor |
Relapse |
Leukemia-free survival |
Overall survival |
GvHD-free, relapse-free survival |
||||
|---|---|---|---|---|---|---|---|---|
|
HR |
p value |
HR |
p value |
HR |
p value |
HR |
p value |
|
|
FB4 vs FT14 |
1.83 |
0.01 |
1.52 |
0.012 |
1.65 |
0.009 |
1.48 |
0.03 |
|
Age |
1.04 |
0.61 |
1.13 |
0.049 |
1.17 |
0.023 |
— |
— |
In line with the previous subset analysis in active AML, treosulfan was associated with superior OS compared with FB4. Notably, the dose of treosulfan used in this study was higher than currently recommended (FT10). Further retrospective studies will be needed to confirm the optimal conditioning regimen.
Treosulfan- versus thiotepa-based conditioning regimen for haploidentical transplant4
A treosulfan-based regimen was previously shown to reduce NRM in patients with AML undergoing allo-HSCT from matched sibling donors (MSD) and unrelated donors (UD). Francesco Saraceni presented results from a retrospective study4 investigating whether this improvement was observed for patients undergoing transplant from haploidentical donors, which does not have a standard conditioning regimen. Treosulfan-based (Treo) or thiotepa-busulfan-fludarabine (TBF) conditioning were compared as the preparatory regimens (Figure 5).
Figure 5. Study design*
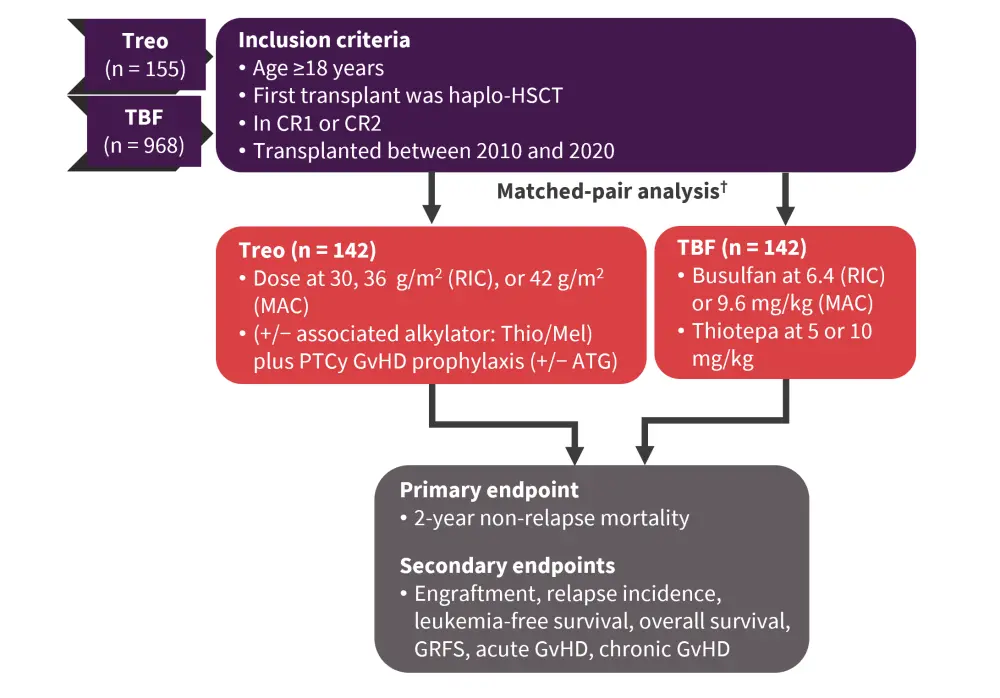
CR1, first complete remission; CR2, second complete remission; GRFS, GvHD-free, relapse-free survival; GvHD, graft-versus-host disease; haplo-HSCT, haploidentical hematopoietic stem cell transplantation; MAC, myeloablative conditioning; RIC, reduced intensity conditioning; TBF, thiotepa-busulfan-fludarabine; Treo, treosulfan.
*Adapted from Saraceni et al.4
Results
Patient characteristics for patients included in the matched-pair analysis are shown in Table 6.
Table 6. Patient characteristics*
|
CR1, first complete remission; RIC, reduced intensity conditioning; TBF, thiotepa-busulfan-fludarabine; Treo, treosulfan. |
||
|
Characteristic |
Treo |
TBF |
|---|---|---|
|
Median age, years (range) |
58 (18.1–75.7) |
58 (21.2–72.2) |
|
Median year of transplant (range) |
2019 (2012–2020) |
2019 (2014–2020) |
|
CR1 at transplant, % |
82 |
82 |
|
RIC, % |
47 |
47 |
|
Stem cell source, % |
||
|
Bone marrow |
13 |
13 |
|
Peripheral blood |
87 |
87 |
|
Cytogenetic risk, adverse, % |
16 |
16 |
Primary and secondary endpoints
- Engraftment was comparable between the two conditioning regimens (95% for both).
- The 2-year primary and secondary endpoint outcomes are summarized in Figure 6.
Figure 6. 2-year survival outcomes*
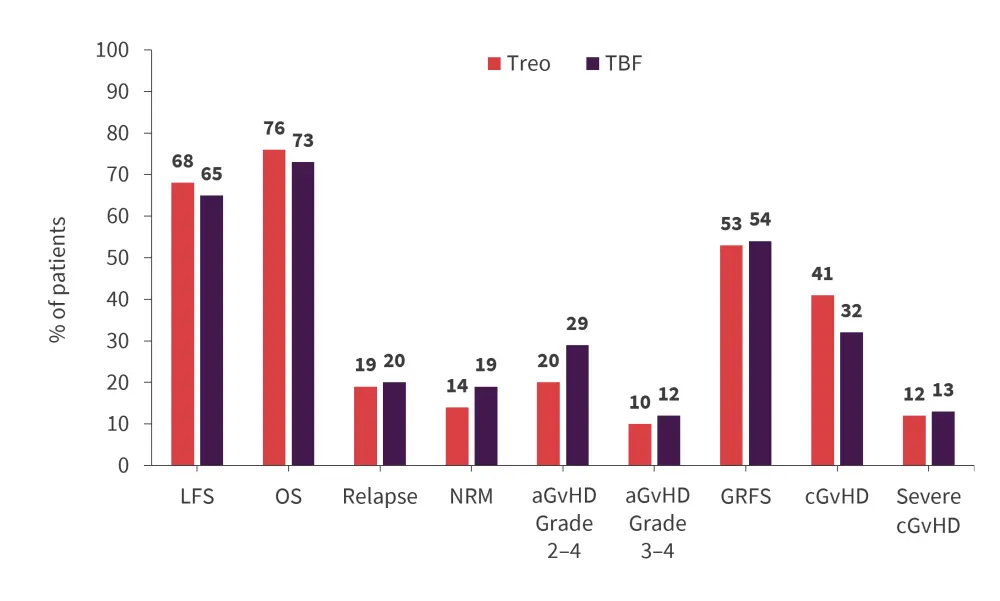
aGvHD, acute GvHD; cGvHD, chronic GvHD; GRFS, GvHD-free, relapse-free survival; GvHD, graft-versus-host disease; LFS, leukemia-free survival; NRM, nonrelapse mortality; OS, overall survival; TBF, thiotepa-busulfan-fludarabine; Treo, treosulfan.
*Adapted from Saraceni et al.4
- There was no significant difference in any of the 2-year survival outcomes between the conditioning regimens.
- When stratifying by conditioning intensity, there was again no significant difference in NRM, relapse incidence, LFS, and OS in patients receiving myeloablative treatment nor reduced intensity conditioning (RIC).
- Finally, the causes of death are shown in Table 7.
- As expected, occurrence of original disease, GvHD, and infection were the most common causes of death in both cohorts.
- Notably, no deaths from veno-occlusive disease were reported in the Treo cohort.
Table 7. Causes of death*
|
GvHD, graft-versus-host disease; SOS, sinusoidal obstruction syndrome; TBF, thiotepa-busulfan-fludarabine; Treo, treosulfan; VOD, veno-occlusive disease. |
||
|
Cause of death, % |
Treo |
TBF |
|---|---|---|
|
Original disease |
41 |
31 |
|
GvHD |
10 |
31 |
|
Infection |
31 |
12 |
|
SOS/VOD |
0 |
6 |
|
Cardiac toxicity |
3 |
3 |
|
Other |
16 |
17 |
In summary, treosulfan and TBF based conditioning regimens produced similar outcomes for patients undergoing haploidentical allo-HSCT. Treosulfan-based conditioning may be a valid alternative in AML, although further studies are needed.
Conclusion
Data from the above studies presented at the 48th Annual Meeting of the EBMT, firstly demonstrate the validity of substituting busulfan with fludarabine in standard conditioning regimens in a relapsed/refractory and complete remission setting; secondly, they demonstrate that the substitution of busulfan with treosulfan led to improved survival outcomes for patients in complete remission when combined with fludarabine. Finally, treosulfan-containing regimens are a valid alternative for patients undergoing haploidentical transplant with similar survival outcomes compared with TBF-based conditioning.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



