All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Clofarabine with fludarabine and busulfan in pretransplant conditioning regimen for AML/MDS: Results from a phase III study
The pretransplant conditioning regimen is an essential component for the long-term outcomes of allogeneic hematopoietic stem cell transplantation (allo-HSCT).1 The AML Hub previously published an article, and an expert opinion interview on conditioning treatment before allo-HSCT.
For patients with acute myeloid leukemia/myelodysplastic syndromes (AML/MDS), the pretransplant conditioning regimen typically consists of the nucleoside analog (NA) fludarabine, combined with the alkylating agent busulfan (intravenous). In human AML cell lines, the later generation NA clofarabine, was found to be more potent than fludarabine, and a combination of clofarabine and fludarabine synergizes to a much higher degree than NA alone when combined with busulfan.2 Further trials determined that a double NA regimen was efficacious and had an acceptable toxicity profile, and that higher doses of clofarabine yield greater antileukemic effects.1,3
Here, we report the key efficacy and safety results from a randomized phase III trial (NCT01471444) of fludarabine + busulfan ± clofarabine as a pretransplant conditioning regimen in high-risk patients with AML/MDS. The results were published by Andersson, et al.1 in the Bone Marrow Transplantation journal, and we provide a summary below.
Study design
This was a randomized phase III clinical trial of 250 patients with AML/MDS. Overall, 120 patients were randomized to receive fludarabine + clofarabine + busulfan (FCB) and 130 patients received fludarabine + busulfan (Flu-Bu). Patients with MDS/AML (n = 22) and myeloproliferative disorders/AML (n = 1) were classified within AML (Figure 1). The inclusion criteria were as follow:
- Patients with AML/MDS aged 3–70 years, in first complete remission (CR), with high-risk cytogenetic features, and/or the need for more than one cycle of chemotherapy to achieve CR.
- Patients with induction-chemotherapy refractory AML, or disease beyond first CR.
- For MDS patients, eligibility allowed an International Prognostic Score System ≥2, or progression after previous chemotherapy.
- Eligibility required an acceptable renal and hepatic function, Karnofsky performance status ≥80, no uncontrolled infection, negative serology for hepatitis B, C, and HIV, and adequate cardiac, and pulmonary function.
- Patients with any hematopoietic cell transplantation-specific comorbidity score (HCT-CI) were included, provided the other criteria were met. HCT-CI scores were dichotomized as 0–2 vs 3–10.
Figure 1. Study design*
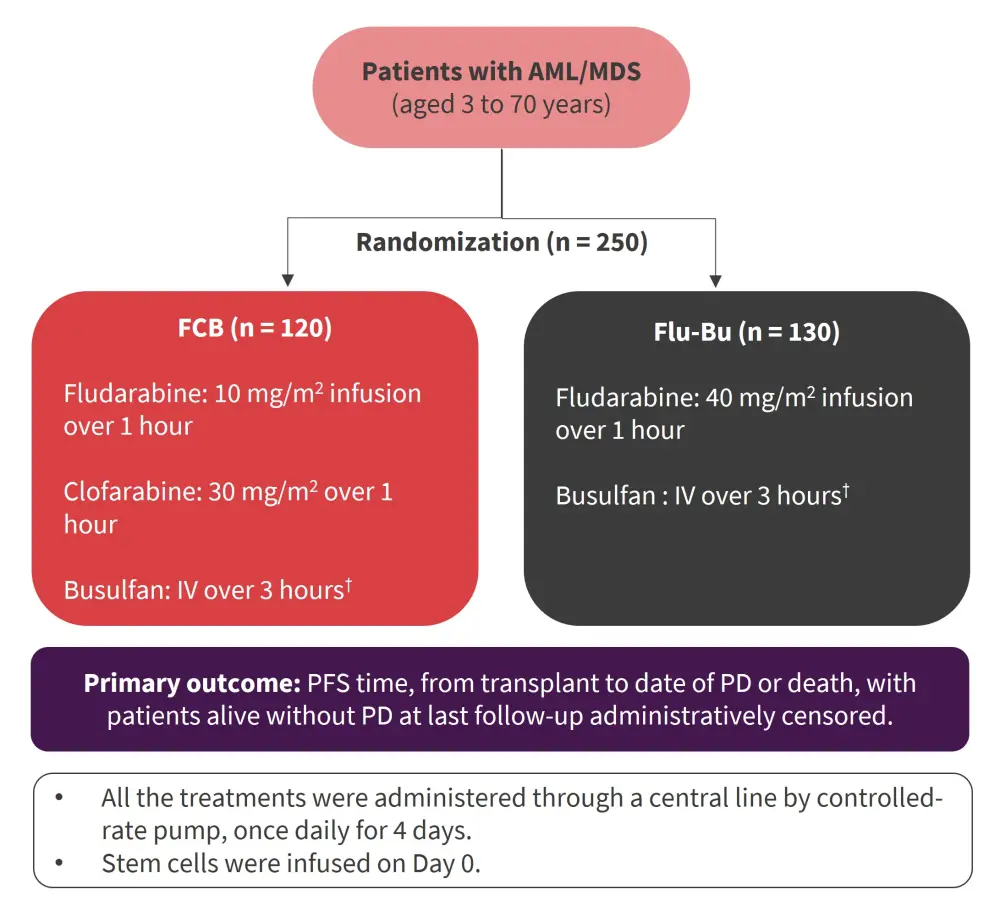
AML, acute myeloid leukemia; AUC, area under the concentration vs time curve; Bu-SE, busulfan systemic exposure; FCB, fludarabine + clofarabine + busulfan; Flu-Bu, fludarabine + busulfan; IV, intravenous; MDS, myelodysplastic syndromes; PD, progressive disease; PFS, progression-free survival.
*Adapted from Andersson, et al.1
†Busulfan was targeted to an average daily Bu-SE, represented by the AUC of 6,000 μMol-min, total course AUC of 24,000 μMol-min ± 5% for patients up to age 60. For patient ages 61–70, the targeted daily Bu-SE was 4,000 μMol-min, a total course of 16,000 μMol-min ± 5%.
Results
Patient characteristics
250 patients with AML (n = 181), and MDS (n = 69) received allo-HSCT. The median age at transplant was 51.4 years. The patient characteristics at pretransplant are summarized in Table 1.
Table 1. Patient characteristics*
|
AML, acute myeloid leukemia; AUC, area under the concentration vs time curve; auto-HSCT, autologous hematopoietic stem cell transplantation; Bu-SE, busulfan systemic exposure; CR, complete remission; FCB, fludarabine + clofarabine + busulfan; Flu-Bu, fludarabine + busulfan; HCT-CI, hematopoietic cell transplantation-specific comorbidity index; HLA, human leukocyte antigens; MDS, myelodysplastic syndromes. |
|||
|
Characteristic, % |
Overall |
FCB |
Flu-Bu |
|---|---|---|---|
|
Gender, n = 250 |
|||
|
Female |
43.6 |
45.8 |
41.5 |
|
Race, n = 243 |
|||
|
Asian |
2.9 |
2.6 |
3.2 |
|
Black |
3.3 |
2.6 |
4.0 |
|
Hispanic |
4.1 |
1.7 |
6.3 |
|
White |
87.2 |
90.6 |
84.1 |
|
Other |
2.5 |
2.6 |
2.4 |
|
Median age, years (range; SD), |
51.4 |
51.9 |
50.9 |
|
≤60 |
71.6 |
71.7 |
71.5 |
|
>60 |
28.4 |
28.3 |
28.5 |
|
Diagnosis, n = 250 |
|||
|
AML |
72.4 |
71.7 |
73.1 |
|
MDS |
27.6 |
28.3 |
26.9 |
|
Treatment-related MDS, n = 69 |
|||
|
No |
81.2 |
73.5 |
88.6 |
|
Yes |
18.8 |
26.5 |
11.4 |
|
Treatment-related AML, n = 181 |
|||
|
No |
85.6 |
89.5 |
82.1 |
|
Yes |
14.4 |
10.5 |
17.9 |
|
Cytogenetic risk category, n = 249 |
|||
|
Poor |
37.3 |
32.8 |
41.5 |
|
Intermediate/good |
62.7 |
67.2 |
58.5 |
|
Median number of chemotherapy regimens (SD), n = 250 |
1.47 (0.85) |
1.46 (0.80) |
1.48 (0.89) |
|
Prior auto-HSCT, n = 250 |
|||
|
0 |
98.8 |
98.3 |
99.2 |
|
1 |
0.8 |
0.8 |
0.8 |
|
2 |
0.4 |
0.8 |
0 |
|
CR, n = 250 |
53.2 |
53.3 |
53.1 |
|
Karnofsky performance, n = 243 |
|||
|
70 |
1.2 |
0.8 |
1.6 |
|
80 |
11.1 |
10.2 |
12.0 |
|
90 |
49.0 |
44.9 |
52.8 |
|
100 |
38.7 |
44.1 |
33.6 |
|
Bu-SE AUC, n = 250 |
|||
|
4,000 |
30.8 |
30.0 |
31.5 |
|
6,000 |
69.2 |
70.0 |
68.5 |
|
Allotype/relation, n = 250 |
|||
|
HLA-identical sibling |
38.0 |
38.3 |
37.7 |
|
Unrelated |
62.0 |
61.7 |
62.3 |
|
Match 10/10 |
60.4 |
58.3 |
62.3 |
|
Match 9/10 |
1.6 |
3.3 |
0 |
|
HCT-CI score, n = 250 |
|||
|
0–2 |
42.8 |
42.5 |
43.1 |
|
≥3–10 |
57.2 |
57.5 |
56.9 |
Clinical outcomes
Overall, 248 patients who underwent allo-HSCT were evaluated. Full donor chimerism was achieved in 92.4% and 86.2% of patients in the FCB group and Flu-Bu group, respectively. Overall, 97.6% of patients remained in, or achieved, CR following the transplant. The median follow-up time for all patients was 66 months. Outcomes were as follows:
- Median progression-free survival (PFS) was 39 months and 28 months in the FCB and Flu-Bu groups, respectively.
- Median overall survival (OS) was not reached in the FCB group and was 54 months in the Flu-Bu group.
- The estimated 3-year PFS probabilities were 52% and 48% in the FCB and Flu-Bu groups, respectively.
- The estimated 3-year OS probabilities were 57% and 53% in the FCB and Flu-Bu groups, respectively.
The median graft-versus-host disease (GvHD)-free, relapse-free survival (GRFS) for FCB was 9.7 months and for Flu-Bu was 9.1 months (p = 0.896). 26 patients died due to non-relapse causes in the FCB group, and 13 patients died in the Flu-Bu group, most commonly from infection and GvHD. Serious adverse events are summarized in Table 2.
Table 2. Serious adverse events by grade and treatment group*
|
DAH, diffuse alveolar hemorrhage; FCB, fludarabine + clofarabine + busulfan; Flu-Bu, fludarabine + busulfan; PRES, posterior reversible encephalopathy syndrome; SOS, sinusoidal obstruction syndrome; VOD, hepatic veno-occlusive disease. |
||||||||
|
Serious adverse event, n |
FCB (n = 120) |
Flu-Bu (n = 130) |
||||||
|---|---|---|---|---|---|---|---|---|
|
Grade 3 |
Grade 4 |
Grade 5 |
All |
Grade 3 |
Grade 4 |
Grade 5 |
All |
|
|
Mucositis |
19 |
0 |
0 |
115 |
25 |
0 |
0 |
119 |
|
VOD/SOS |
2 |
0 |
1 |
3 |
0 |
0 |
0 |
0 |
|
Elevated bilirubin |
6 |
1 |
0 |
49 |
7 |
0 |
0 |
42 |
|
PRES |
3 |
0 |
0 |
3 |
1 |
0 |
0 |
1 |
|
DAH |
0 |
1 |
1 |
2 |
0 |
0 |
2 |
2 |
|
Pneumonitis |
3 |
1 |
1 |
5 |
5 |
0 |
1 |
6 |
|
Hand/foot syndrome |
1 |
0 |
0 |
11 |
0 |
0 |
0 |
11 |
|
Infection |
||||||||
|
Bacterial |
21 |
3 |
6 |
37 |
11 |
3 |
1 |
33 |
|
Viral |
9 |
0 |
3 |
55 |
4 |
1 |
2 |
71 |
|
Fungal |
4 |
2 |
3 |
9 |
2 |
2 |
2 |
6 |
Non-relapse mortality estimates are displayed in Figure 2.
- The cumulative 1-year relapse incidences (RI) were 18% and 35% for the FCB and Flu-Bu groups, respectively.
- The cumulative 3-year RI were 25% and 39% for the FCB and Flu-Bu groups, respectively (p = 0.02).
When RI was evaluated according to disease status at transplant, the benefits of FCB were larger for not in complete remission (NCR) patients; this benefit was offset by the higher non-relapse mortality rates, and only the NCR patients >60 years maintained the PFS and OS benefits. Patients with lower HCT-CI score (0–2) benefitted the most from FCB for both PFS and OS.
Figure 2. Cumulative non-relapse mortality*
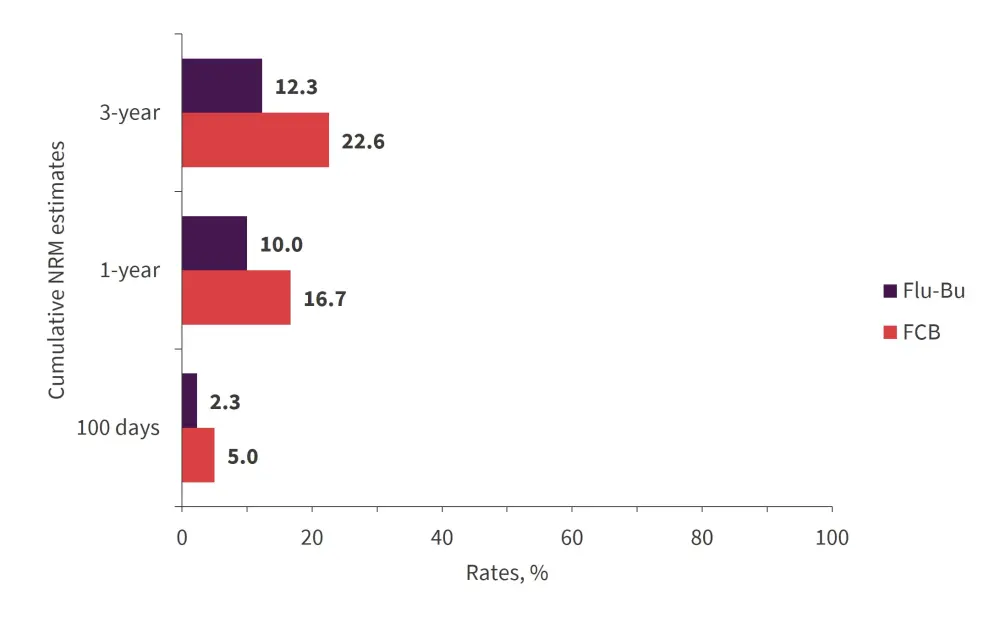
FCB, fludarabine + clofarabine + busulfan; Flu-Bu, fludarabine + busulfan; NRM, non-relapse mortality.
*Adapted from Andersson, et al.1
Conclusion
Conditioning with FCB did not improve PFS overall; nevertheless, it showed better outcomes in NCR patients, or patients >60 years. In patients with active AML and low HCT-CI (0–2), FCB performed better than Flu-Bu; however, HCT-CI was not considered during the design of this study, and therefore, the results regarding HCT-CI should be considered non-confirmatory and require further investigation. Andersson, et al. recommended that the FCB treatment should be personalized based on CR/NCR status, and by including a risk assessment for treatment-related complications based on the HCT-CI and performance scores. Overall, the FCB treatment has shown to be a promising option for older patients (aged <70) with AML/MDS, and may yield improved disease control.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

