All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Bemcentinib in patients with HR-MDS or AML who are R/R to HMAs: Results from the phase II BERGAMO trial
Context
Hypomethylating agents (HMAs) are often used in the treatment of higher-risk myelodysplastic syndromes (HR-MDS), or in combination with venetoclax in patients with acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy or allogeneic hematopoietic stem cell transplantation.1 However, many patients experience either non-response to HMAs or relapse.1 The phase II BERGAMO trial investigated the safety and efficacy of bemcentinib, an oral, selective AXL inhibitor, in patients with either HR-MDS or AML who were ineligible for intensive chemotherapy or allogeneic hematopoietic stem cell transplantation and were relapsed/refractory following treatment with HMAs.1 Kubasch et al.,1 recently published results from this trial in Leukemia, which we are pleased to summarize below.
Study design and patient population
The phase II BERGAMO trial (NCT03824080) included 45 patients (MDS = 18 and AML = 27) treated at 10 sites in Germany and France within the European Myelodysplastic Neoplasms Cooperative Group:
- Patients received an initial dose of 400 mg of bemcentinib once daily on Days 1–3 of Cycle 1, and a maintenance dose of 200 mg on Days 4–28 of Cycle 1 and in subsequent 28-day treatment cycles.
- The primary endpoint was the overall hematological response rate, defined as complete remission, marrow complete remission, partial response, stable disease, or hematologic improvement assessed at Week 17 after four treatment cycles.
- Secondary endpoints included toxicity, overall survival, progression-free survival, time to treatment failure, duration of response, and best overall response.
- The median age was 79 years (range, 62–86).
Key findings1
Response to bemcentinib
The overall hematological response rate was 24%, with a greater proportion of patients with HR-MDS responding than patients with AML (Figure 1).
Figure 1. Response rates at Week 17 after four cycles of bemcentinib in the phase II BERGAMO trial*
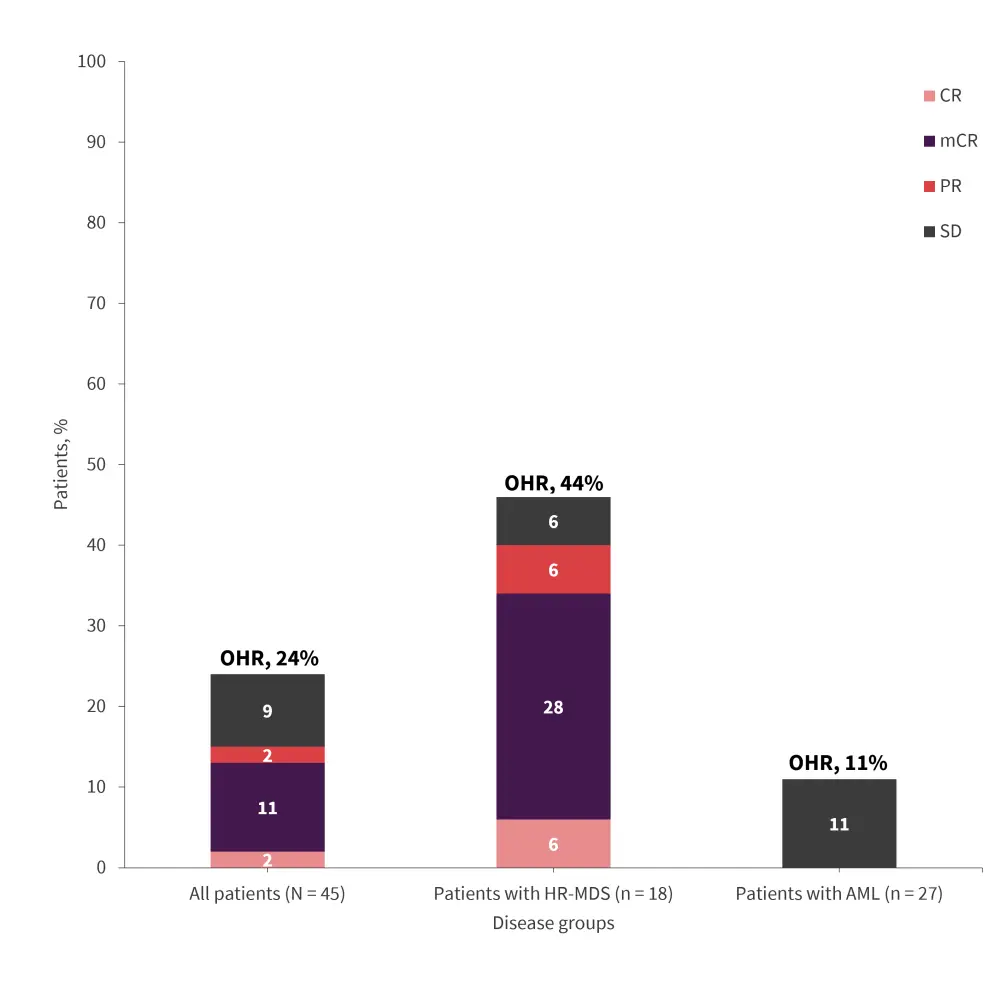
AML, acute myeloid leukemia; CR, complete remission; HR-MDS, higher-risk myelodysplastic syndromes; mCR, marrow CR; OHR, overall hematological response; PR, partial remission; SD, stable disease.
*Data from Kubasch, et al.1
- Median number of treatment cycles was four (range, 3–21 cycles).
- Median duration of treatment was 10 weeks (range, 1–95 weeks).
- Among patients with MDS with a marrow complete remission (n = 5), 80% had a normal karyotype and 20% had Y chromosome loss.
- Among patients with MDS who were non-responders (n = 10), 40% had complex karyotype.
- Median duration of response was 25 and 12 weeks in patients with MDS and AML, respectively.
- Best overall response for the complete study period was 56% and 15% for patients with MDS and AML, respectively.
Survival outcomes
- One-year overall survival rate was 28% overall and 54% in patients with MDS.
- One-year progression-free survival rate was 8% and 6% in patients with AML and MDS, respectively.
- The median time to event was 8 weeks in patients with AML vs 22 weeks in patients with MDS (p = 0.012).
Safety
- At the data cutoff, bemcentinib-related Grade 3–5 serious adverse events occurred in 31% of patients (Table 1).
- In total, 25 patients died, most commonly due to disease progression (n = 18) not related to bemcentinib.
Table 1. Bemcentinib-related Grade 3–5 serious adverse events reported in the BERGAMO trial*
|
*Data from Kubasch, et al.1 |
|
|
Serious adverse events, n |
All patients (N = 45) |
|---|---|
|
Grade 5 |
3 |
|
Disease progression |
2 |
|
Acute kidney injury |
1 |
|
Grade 4 |
0 |
|
Grade 3 |
14 |
|
Sepsis |
2 |
|
Acute kidney injury |
1 |
|
Abdominal pain |
1 |
|
Bone pain |
1 |
|
Febrile bone marrow aplasia |
1 |
|
Febrile neutropenia |
1 |
|
General physical health deterioration |
1 |
|
Headache |
1 |
|
Nausea |
1 |
|
Periodontitis |
1 |
|
Pneumonia |
1 |
|
Pneumonitis |
1 |
|
Upper gastrointestinal hemorrhage |
1 |
Exploratory analysis
- STAG2 mutations were frequently observed in responders (40%) compared with non-responders (8%; p = 0.048).
Key learnings
In the phase II BERGAMO trial, bemcentinib had a good tolerability profile, with modest single-agent activity in the patient population. Response rate was greater in patients with HR-MDS than those with AML and also in patients with STAG2 mutations, warranting further investigation.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

