All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MDS Alliance.
The mds Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mds Hub cannot guarantee the accuracy of translated content. The mds and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MDS content recommended for you
Allo-HSCT in patients with high-risk MDS: Analysis from the BMT CTN 1102 trial
Context
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative treatment for patients with myelodysplastic syndromes (MDS).1 To assess the impact of the presence of high-risk mutations on outcomes following allo-HSCT, Versluis et al.1 performed a genetic analysis of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 1102 trial. Findings were recently published in the Journal of Clinical Oncology which we are pleased to summarize below.
Study design and patient population
- Targeted DNA sequencing using a variant allele fraction cutoff of 0.02 was performed on blood samples from 309 out of 384 patients from the multicenter phase II BMT CTN 1102 study (NCT02016781). The study compared reduced intensity conditioning allo-HSCT with hypomethylating therapy or best supportive care in patients aged 50–75 with International Prognostic Scoring System intermediate-2 or high-risk de novo MDS.
- Baseline characteristics and mutation frequency were similar between patients who underwent allo-HSCT (donor arm) and those who did not (no-donor arm) (Table 1).
- Median follow-up in survivors was 32 months (range, 6–38 months).
Table 1. Most common somatic gene mutations in patients in the BMT CTN 1102 trial with samples available*
|
BMT CTN, Blood and Marrow Transplant Clinical Trials Network. |
|||
|
Gene mutation frequency, % |
p value |
||
|---|---|---|---|
|
≥ 1 mutation |
88 |
|
|
|
TP53 |
28 |
29 |
0.89 |
|
ASXL1 |
23 |
29 |
0.37 |
|
SRSF2 |
16 |
16 |
0.99 |
|
DNMT3A |
17 |
10 |
0.20 |
Key findings
- Patients with TP53 or KMT2A-partial tandem duplication (PTD) mutations had inferior 3-year overall survival (OS) compared with patients without these mutations (univariate analysis; Figure 1).
Figure 1. 3-year OS by gene mutations present in patients in the BMT CTN 1102 trial*
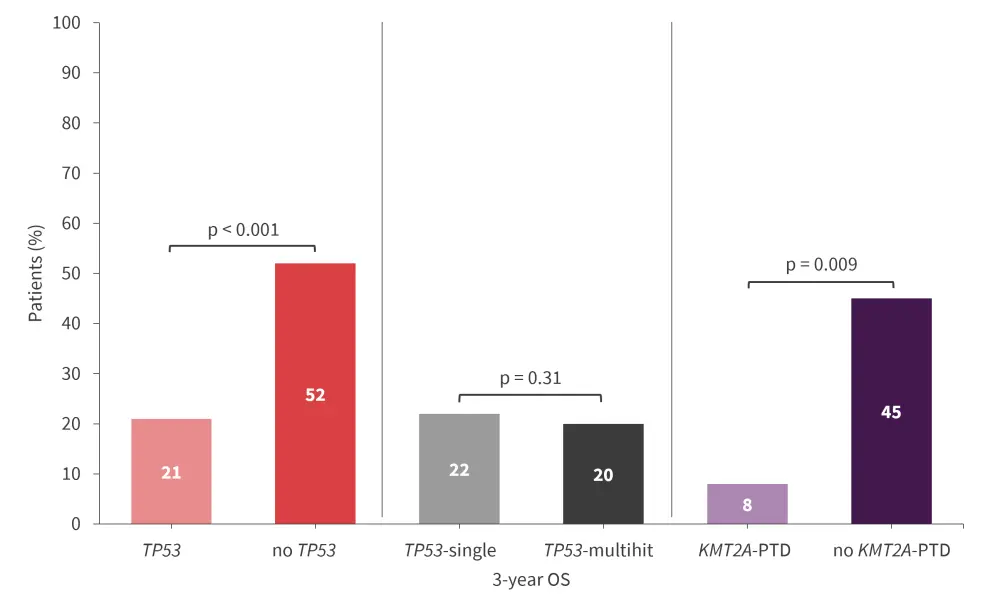
BMT CTN, Blood and Marrow Transplant Clinical Trials Network; OS, overall survival; PTD, partial tandem duplication.
*Data from Versluis et al.1
Multivariable analysis found (Figure 2):
- TP53, KMT2A-PTD, and DDX41 mutations were associated with OS.
- Patients in the donor arm had improved OS vs the no donor arm.
- The allelic state of TP53 mutations was associated with inferior outcomes.
In addition, allo-HSCT time-dependent covariate analysis revealed:
- an association between the presence of TP53 or KMT2A-PTD mutations, and inferior OS;
- patients who received allo-HSCT (n = 197) also had a lower instantaneous risk of death when compared to patients who did not (n = 78; hazard ratio, 2.31; 95% confidence interval, 1.53–3.49; p < 0.001); and
- in patients with TP53 mutations, OS was improved in those who underwent HSCT vs those who did not (hazard ratio, 3.89; 95% confidence interval, 1.87–8.12; p < 0.001; multivariable analysis).
Figure 2. A: Multivariable analysis of OS (donor vs no donor), after adjustment for covariates. B: Multivariable analysis of OS (allo-HSCT as a time-dependent covariate)*
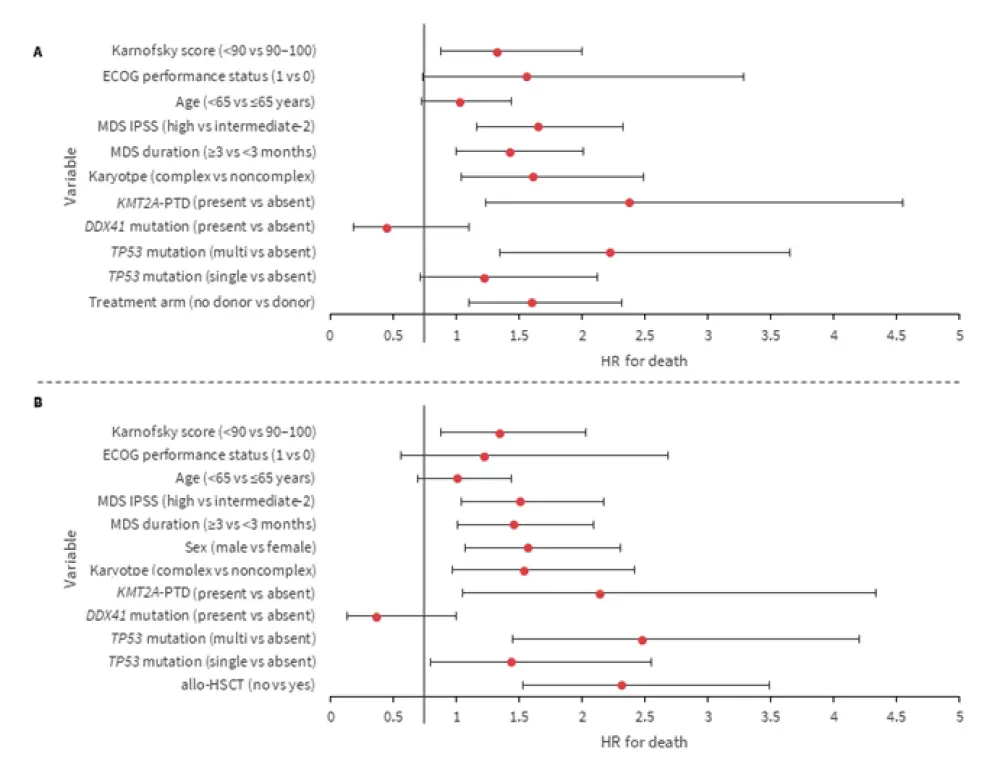
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; ECOG, Eastern Cooperative Oncology Group; HR; hazard ratio; IPSS; International Prognostic Scoring System; MDS, myelodysplastic syndromes; OS, overall survival; PTD, partial tandem duplication.
*Adapted from Versluis et al.1
Key learnings
Allo-HSCT can be beneficial in patients with high-risk MDS, including patients with TP53 mutations.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


